
Also, Isogen completes sterile facility; Dishman appoints head of generic API business; FDA releases final rule on authorized generic drugs and NDA submissions; more...

Also, Isogen completes sterile facility; Dishman appoints head of generic API business; FDA releases final rule on authorized generic drugs and NDA submissions; more...

The US Food and Drug Administration last week posted the final version of its Guidance for Industry, ANDAs: Impurities in Drug Substances.

Also, Gilead and Tibotec form agreement; FDA approves 2009-2010 flu vaccine; Catalent appoints VP in packaging unit; more...

Drugmakers have the common goal of manufacturing safe and sterile pharmaceutical products and understand that the filtration process is a critical means of achieving this goal. Preuse filter-integrity testing provides evidence that a filter will perform correctly, has the right pore size, and has been installed correctly.

Also, Lonza and Medarex sign agreement; Covance appoints VP and chief scientific officer of global analytical services; more...

Also, Lundbeck acquired LifeHealth; FDA is seeking a director of its new tobacco regulation branch; Charles River Labs announces personnel changes; more...

Also, Wyeth and Catalyst sign agreement; FDA seeks public opinion about tobacco regulation; Catalent appoints VP of quality and regulatory affairs; more...

This week, the US Pharmacopeial Convention and the Vietnamese Pharmacopoeia Commission signed a memorandum of understanding that will help ensure the safety of Vietnamese medicines.

The regulators are doing it. But industry's fear of sharing information may leave them behind.

Is it good policy to pay for bad behavior?

Brief pharmaceutical news items for July 2009.

Developers of low-dose drugs in solid oral dosage forms will find theoretical considerations and practical advice in a new book.
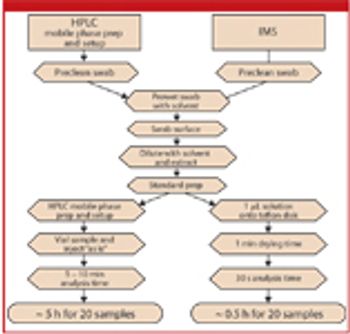
The authors discuss the theory of ion mobility spectrometry, its benefit over HPLC analysis in cleaning verification, and the experimental considerations for method validation and validation.

Also, Adimab forms deals with Merck and Roche; Manhattan Pharmaceuticals' CEO and president steps down; more...

Also, FDA debars clinical investigators; Jubilant Organosys forms deal with Endo Pharmaceuticals; AMRI makes changes to its India management team; more...

Also, TorreyPines Therapeutics to liquidate assets and dissolve company; EU's competition services to examine Pfizer/Wyeth merger; Akorn appoints Raj Rai interim CEO; more...

Consumer-care products, electronics, and select industrial firms comprise AMR Research's Supply Chain Top 25 for 2009, offering an opportunity for the pharmaceutical industry to examine best practices in supply-chain management.

Also, WACKER expands Iowa facility; EMEA releases a Q&A document for PIPs; Metrics consolidates quality operations; more...

A book guides readers through the regulatory requirements for computerized quality systems.
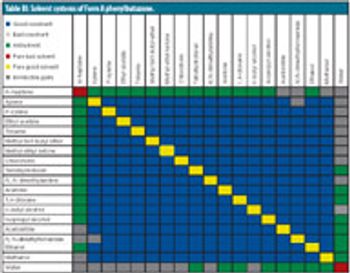
The authors describe the importance of a rapid and an abbreviated screening strategy in initial solvent screening. This article contains bonus online-exclusive material.

Misleading the public about their investments-be it money or medicine-is unacceptable.

It can take a lot of work to make sure nothing happens.
Debates about science, manufacturing, and European regulations will shape the approval process for follow-on biologics in the United States.

Today, approximately 1.5 million counterfeit medicine packs enter the legal supply chain each year - in other words, one pack in every 20000 is counterfeit.

Also, Johnson & Johnson acquires Cougar Biotechnology; NIH launches program for rare and neglected diseases; PPD restructures leadership positions; more...