
The nation's healtcare system needs an overhaul, but it has to be done right.

The nation's healtcare system needs an overhaul, but it has to be done right.

Brief pharmaceutical news items for October 2009.

The author discusses control strategies via near infrared instrumentation for continuous mixing, granulation, drying, and extrusion with a more focused detail on mixing.
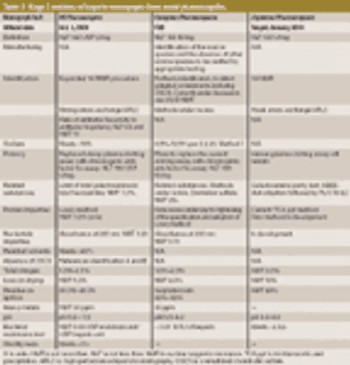
USP workshop participants support new methods to safeguard heparin products but desire international harmonization. This article contains bonus online-exclusive material.
A review of NIPTE's core projects and its plans for training-and retraining-the pharmaceutical industry.

Dr Martin Cockcroft, Operations Manager at Tepnel Pharmaceutical Services, an established business of Gen-Probe Inc., speaks about harmonized microbiology methods.

Company and People Notes: Boehringer Ingelheim will acquire Wyeth's animal health business; Amsterdam Molecular Therapeutics appoints CEO; more...

Company and People Notes: Wyeth and Ambrx form development pact; Elite Pharma appoints CEO and CSO; more...

The US Food and Drug Administration sent Bayer Schering Pharma (Berlin) a Warning Letter on Aug. 5, 2009, citing deviations from current good manufacturing practice in the manufacture of nonsterile active pharmaceutical ingredients (APIs).

Company and People Notes: Sanofi Pasteur signs H1N1 vaccine deal with Brazilian government; Helsinn Group appoints CEO of US business; more...

Company and People Notes: Neoprobe and Laureate Pharma form manufacturing agreement; Akela Pharma appoints CEO and chairman; more...

Also, Orexo and Novartis form agreement; Affitech appoints Robert Burns CEO; more...

Brief pharmaceutical news items for September 2009.

After years of promomting QbD concepts, FDA's ready to take action on nonconformers.

As new process validation guidelines emerge, industry needs to reinvent how it releases product.

IPEC's new stability testing guide takes into account the full supply chain's storage conditions.

When validating automated systems from third-party providers, using the V model and failure modes effects and criticality analysis (FMECA) early in the process can help.

Company and People Notes: UCB and Novartis form agreement; AAIPharma appoints VP of regulatory affairs; more...

Also, XCELERON and GSK form agreement; Millipore appoints VP of life sciences; more...

Also: DSM's North Carolina facility receives SafeBridge certification; Dynavax CFO to retire; more...

Also, Pfizer forms two research agreements in China; NanoInk appoints John Kubricky to its scientific advisory board; more...

The US Food and Drug Administration and European Medicines Agency (EMEA) launched this week a joint initiative to collaborate on international good clinical practice (GCP) inspection activities.
Editors' Picks of Pharmaceutical Science & Technology Innovations

Brief pharmaceutical news items for August 2009.

Biotech firms must first close the gaps between science and biology on the path toward QbD.