
Readers can learn about the importance of measuring and controlling water activity in a comprehensive new book.

Readers can learn about the importance of measuring and controlling water activity in a comprehensive new book.

Particularly in a more connected world, individual contributions can make a difference.

Editors' Picks of Pharmaceutical Science & Technology Innovations

Dumbed-down presentations and poor speaker selections are destroying a valuable industry tool.

With so many healthcare and pharmacy websites, consumers could use the agency's nod of support.

The quality assurance environment is forcing pharmaceutical companies to face new challenges. In light of this, the authors conducted a study of professionals from some of the world's top pharmaceutical companies to identify key QA concerns.

Manual sample preparation methods for solid dose pharmaceuticals have a number of inherent disadvantages, primarily because they are time consuming and unpredictable.

Company and People Notes: Merck KGaA to build R&D hub in Beijing; Depomed appoints VP of operations; more...

Company and People Notes: Genzyme provides updates on enzyme replacement products and FDA complete response letter; Watson appoints VP of global operations; more...

Company and People Notes: 3M forms agreement with VaxInnate; TRIN Pharma appoints CEO; More...

The American Association of Pharmaceutical Scientists (AAPS) recognized researchers at the organization's 2009 Annual Meeting and Exposition in Los Angeles this week.

The Society of Chemical Manufacturers and Affiliates (SOCMA) reported last week that the US Food and Drug Administration responded to the association's citizen petition relating to the inspection process of foreign drug-manufacturing facilities.

Company and People Notes: sanofi aventis forms pact with Micromet; Laureate Pharma adds members to its business team.

Last week, the steering committee and expert working groups of the International Conference on Harmonization (ICH) met in St. Louis, Missouri. A major success of the meeting was the adoption of several annexes to ICH Q4B on pharmacopeial texts.

A panel of industry and regulatory experts, including FDA, discuss efforts to secure the global pharmaceutical supply chain. This article contains bonus online-exclusive material.

While Congress debates hundreds of healthcare plan proposals, perhaps we, the public, can get in the game too.

The authors developed a robust, automated system to conduct Karl Fischer moisture assays for lyophilized products.

FDA's Edwin Rivera-Martinez on dodging supply-chain challenges.
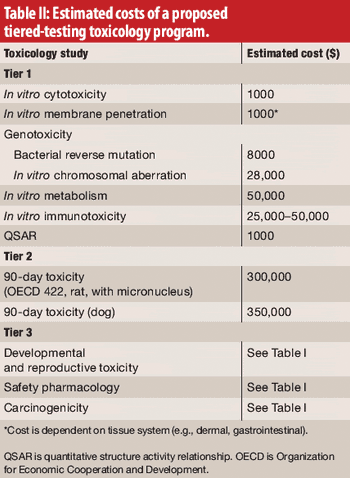
The authors, representing the International Pharmaceutical Excipients Council, propose a new evaluation procedure, including tiered toxicology testing for excipients.

Editors' Picks of Pharmaceutical Science & Technology Innovations

The authors used a light-transmission-based static division system to detect particles of foreign contaminants in prefilled vials.

Also, Astellas and Medivation sign development pact; WuXi PharmaTech appoints VP of business development, more...

Company and People Notes: SurModics forms agreement with Roche and Genentech; Hospira names Daphne Jones senior VP and chief information officer; more...

The European Fine Chemicals Group (EFCG) gave an update of some of its activities at a press conference held at CPhI Worldwide (Spain) last week, and revealed some disturbing facts regarding counterfeit APIs, which the group is particularly concerned about.

Company and People Notes: Bayer Schering Pharma forms collaboration with Compugen; Takeda San Francisco appoints VP of process science; more...