
Also, UCB will divest certain business in emerging markets to GSK; the KineMatik Group named Michael G. Jarjour president and CEO; more...

Also, UCB will divest certain business in emerging markets to GSK; the KineMatik Group named Michael G. Jarjour president and CEO; more...

Also, Elan explores strategic alternatives; NanoGuardian appoints John D. Glover to lead its Security Advisory Board; more...

The US Food and Drug Administration issued its Draft Guidance for Industry on Submission of Laboratory Packages by Accredited Laboratories.

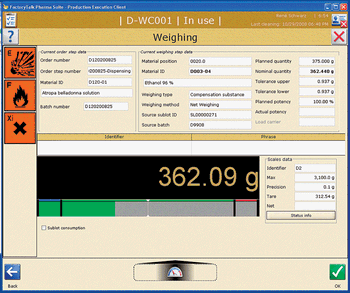
An integrated information technology environment can help pharmaceutical companies find new processes and approaches to improve manufacturing efficiency, productivity, and quality.

Also, Bristol-Myers Squibb forms collaboration with ZymoGenetics, Hana Biosciences appoints VP of regulatory affairs, more...

Also, Novozymes Biologicals settles pollution case with the US Department of Justice; EntreMed restructures management team; more...

Michigan State University researchers have discovered a technique for viewing whole cells to gain an understanding of protein inclusion bodies.
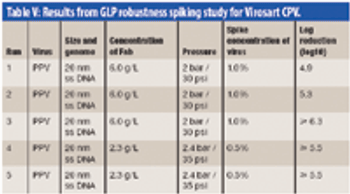
The authors examine the challenges of integrating a large-scale chromatography and nanofiltration process for purification of a polyclonal antibody.
Editors' Picks of Pharmaceutical Science & Technology Innovations

The incoming administration has renewed hope for stem cells, but less adequate copycats may follow.

Brief pharmaceutical news items for January 2009.
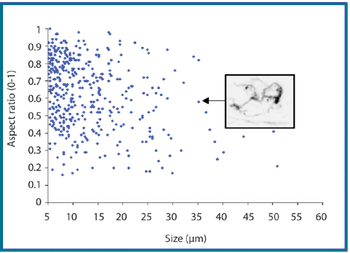
The authors describe a novel analytical approach that uses the shape-analysis capabilities of MFI to detect and enumerate silicone oil microdroplets in protein formulations that also contain aggregates of similar size and in a similar concentration.
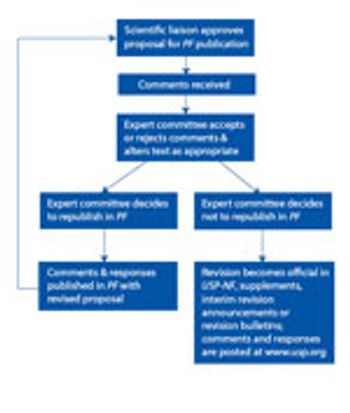
The USP public-comment process exists for a reason. Industry needs to take advantage. This article contains bonus online-exclusive material.

Instead of hampering progress, cost pressures might inspire Big Pharma to get creative.

Also, Crucell and DSM announce deals with GSK, Talecris, and CSL; Nobel Prize winner Luc Montagnier joins Viral Genetics; more...

The US Food and Drug Administration issued a draft guidance, Genotoxic and Carcinogenic Impurities in Drug Substances and Products: Recommended Approaches.

Also, BASF opens lab in Mumbai; Evotek president and CEO to resign; more...

At its annual business briefing held last week, Merck & Co. outlined its short- and long-term strategy for growth. Its strategy is focused on increased penetration in emerging markets, the establishment of a business for developing follow-on biologics or biosimilars, and a new commercial model for product life-cycle management.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the December 2008 edition from Spiroflow Systems and Sepha.

Also, NicOx and DSM make manufacture and supply pact for naproxcinod drug substance; NovaRx appointed Norrie Russell president and COO; more...

A Centers for Disease Control and Prevention (CDC) study confirmed that severe adverse reactions reported in the fall and winter of 2007 resulted from heparin contaminated with oversulfated chondroitin sulfate (OSCS).

Also, AstraZeneca announces changes to its supply chain operations; Christian Velmer appointed head of Wyeth Canada; More...

The authors evaluated the effect of polymer composition on the drug-release profile and the effect of storage conditions on dissolution characteristics.

The past year saw major acquisitions attempted, completed, rejected, and stalled.

Readers provide insight into the best companies to work for as well as the ups and downs of their jobs.