Pharmaceutical Technology is pleased to recognize the winners of its Innovations in Pharma Science Awards.

Pharmaceutical Technology is pleased to recognize the winners of its Innovations in Pharma Science Awards.

Also, Johnson & Johnson will acquire Omrix Biopharmaceuticals for $438 million; Charles River Laboratories promoted Foster Jordan to corporate senior VP of endotoxin and microbial detection products; more...

The American Association of Pharmaceutical Scientists (AAPS) recognized researchers in the pharmaceutical sciences at AAPS Annual Meeting and Exposition in Atlanta last week.

Also, BASi opens European office in Warwickshire, UK; Acusphere's Howard Bernstein Resigns; More...

US marshals seized 11 lots of heparin from Celsus Laboratories (Cincinnati, OH) at the request of the US Food and Drug Administration.

Also, Patheon opens new European headquarters, Cynvec appoints Frank D. Stonebanks president and CEO, more...

PAT may reduce costs by helping companies control process variability, improve yields, reduce waste, and produce high-quality therapies consistently. Companies that have not yet embraced PAT may find its potential to reduce expenses a compelling argument in its favor during this time of financial difficulty.

Until recently, automated inspection of solid-dose pharmaceuticals was crude, expensive, slow, and difficult to change over. But technological advances have ushered in a new class of vision-inspection systems that is more effective and more commercially viable than older systems.

Also, Sartorius Stedim Biotech GmbH to acquire Wave Biotech; AstraZeneca's John Patterson to retire; more...

Brief pharmaceutical news items for November 2008.

Attendees at a recent workshop endorsed new methods to detect metals in drugs, dietary supplements, and food ingredients.

Pharma companies could benefit from the lessons learned in this fall's financial crisis.

Pharmaceutical Technology will feature video coverage of AAPS this month.

Molecules called "chaperones" facilitate correct protein folding.
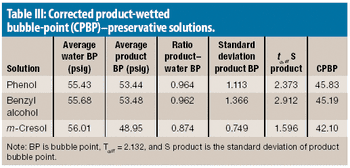
The authors explain the factors that can cause a failure in a bubble-point integrity test and what to consider when a product-specific bubble point must be defined.
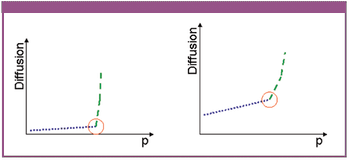
The authors describe a novel approach for the integrity testing of large sterile filter systems such as multiround housings and describe a multipoint diffusion test capable of detecting minor failures.
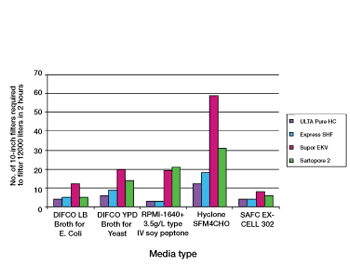
Proper selection of normal flow filters leads to increased process efficiency from early phase product development through to full-scale biopharmaceutical production.
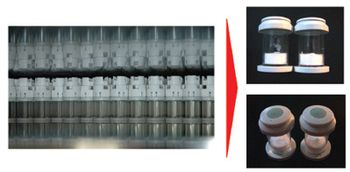
The authors present an aseptic-filling process for freeze-dried liquids using the closed-vial technology.
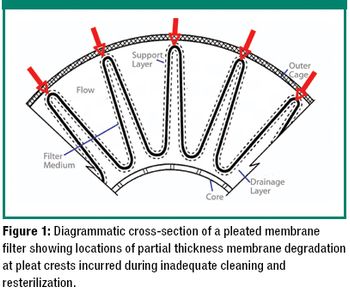
The author examines re-use of hydrophilic- or hydrophobic-membrane sterilizing-grade filters in liquid sterilizing applications.
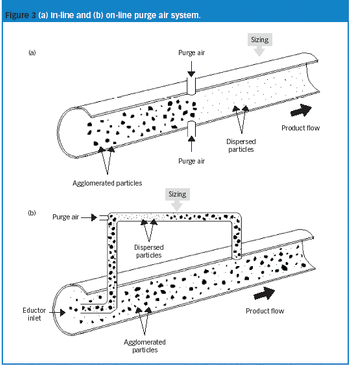
FDA advocates building quality into a product through PAT.

Efficiency is more than just a buzzword in today's pharmaceutical industry; declining productivity and diminishing returns on investment have made it an over-arching mindset that is critical to corporate survival.

Also, Maxygen looks to costs, jobs; Receptor BioLogix appoints Dale R. Pfost CEO; more...

Pfizer and UCB formed a technology company named Cyclofluidic with the aim of accelerating the drug-discovery process.

Also, MedImmune opens Cambridge, UK, facility and makes reverse engineering pact with Omninvest; BD Medicine appoints Carol Adiletto VP of clinical and regulatory affairs; more...

Also, Millipore opens new membrane-casting manufacturing facility in Ireland; Surface Logix appoints Keith Dionne president, CEO, and a member of the board; more...