
The president of BIO proposes the ingredients needed for industry growth.

The president of BIO proposes the ingredients needed for industry growth.
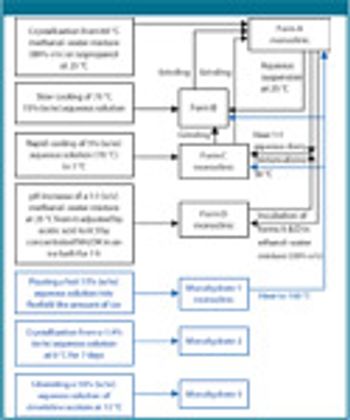
The authors describe the importance of a rapid and an abbreviated screening strategy by initial solvent screening in 20-mL scintillation vials.

Manufacturers of therapeutic monoclonal antibodies consider new paradigms in purification technologies.

The organizations' presidents discuss market exclusivity, approval processes, and pending legislation.

Technology can solve enterprise-level problems.

With pressure to cut costs, shorten the pipeline life cycle and maximize return on investment, pharmaceutical manufacturers need tools that help them improve enterprise-wide communications, reach critical decisions faster and produce timely, accurate reports on how compounds are progressing.

Identification of fungi, especially filamentous fungi, has been a very difficult task. Because of the amount of experience required to accurately identify filamentous fungi to the species level, it has become acceptable to either identify these organisms to the genus level or, in some cases, simply identify them as "molds."

Also, Roche's Avastin trial does not meet endpoint; Innate Pharma names regulatory VP; more...

The US Food and Drug Administration has agreed to address some core industry questions regarding the changes made to US Pharmacopeia General Chapter <467> in July 2008.

The US Food and Drug Administration finalized a Guidance for Industry this week that aims to clarify the submission of new drug applications (NDAs) and biologics license applicants (BLAs) using the common technical document (CTD) format, including the electronic CTD (eCTD).

Also: Sanofi-aventis acquires BiPar Sciences; FDA issues Warning Letter to Chinese heparin manufacturer; Halo Pharmaceutical appoints chief scientific officer; more...

The United States Pharmacopeial (USP) Convention signed a memorandum of understanding (MOU) with the Federal Service on Surveillance in Healthcare and Social Development of the Russian Federation (Roszdravnadzor) last week in Moscow.

Also, Sanofi-aventis acquires Medley and Laboratorios Kendrick; Eli Lilly's Cook to retire from board; more...

Pfizer outlined the progress of its late-stage pipeline last week and announced this week plans to separate its research activities into two organizations, one focused on small molecules and the other on biologics, pending the closure of its pending acquisition with Wyeth.

Also, Genzyme and Bayer HealthCare form agreement; FDA releases draft guidances; TransMolecular appointed Robert Radie president and CEO
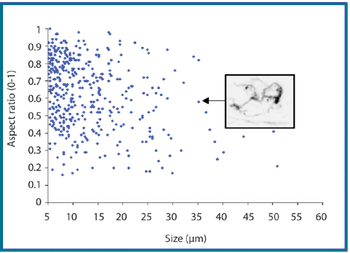
The authors describe a novel analytical approach that uses the shape-analysis capabilities of MFI to detect and enumerate silicone oil microdroplets in protein formulations that also contain aggregates of similar size and in a similar concentration.

Also, SOCMA changes name; two FDA approvals; Biogen Idec names chief operating officer; more...
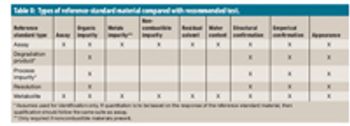
The author reviews the types of reference-standard materials used in drug-product manufacturing, discusses current regulatory requirements, and outlines a reference-standard qualification program.

Brief pharmaceutical news items for April 2009.

FDA is poised to gain authority and resources to ensure the quality of food and drugs.
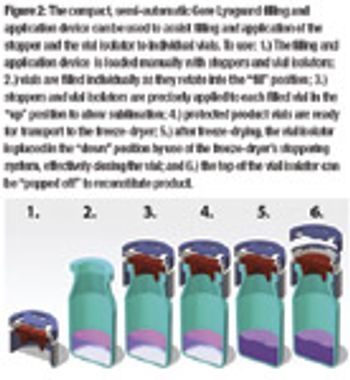
By reducing cycle time and implementing quality-by-design inspired engineering, advanced lyophilization systems are driving the industry toward greater efficiency and control.

Standards data is helpful, but FDA needs to apply its information across the board. This article contains bonus online-exclusive material.

Obama's cost-containment and science-innovation initiatives need to overlap.

The financial and economic downturn is likey to have long-term implications for outsourcing.
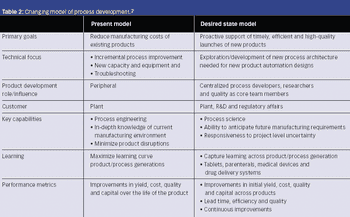
Operational excellence awaits, but only if you can implement PAT successfully.