
Excipient manufacturing site achieves EXCiPACT certification following regulatory inspections.

Excipient manufacturing site achieves EXCiPACT certification following regulatory inspections.

Experts attending the European Psychiatry Association Congress in Vienna say that Adasuve has made an impact in the treatment of agitation in patients suffering from schizophrenia or biopolar I disorder.

The countdown has begun for one of the biggest trade shows for the process industry.

While biotech will revolutionize our industry through new products and new reaction pathways, process analytics is the link between process automation and the laboratory sector, and water plays a key role as a valuable resource all over the world.

Pharmaceutical companies are constantly aiming for shorter drug-development cycles and advances in formulation development produce significant benefits.

Widespread use and abuse of opioid painkillers is prompting efforts to develop new drugs and formulations that resist abuse while providing relief to legitimate patients.

Pharmaceutical Technology spoke with Tim Kearns, pharmaceutical and medical devices manager at Videojet.

Merus and Selexis will combine technology platforms to produce colorectal cancer combination therapy.

Medicago's new production facility will make plant-based vaccines and therapeutics.

Economic benefits, equipment availability, research results, and FDA support are driving progress in continuous processing.

The dinner will be held on Oct. 14, 2015 during CPhI Worldwide, at the Prado Museum in Madrid.

Supply chain consortium establishes a working group to address quality problems in India.

Juno Therapeutics announces that it will expand its cellular therapies pipeline with the acquisition of Stage Cell Therapeutics for €52.5 million.

Pluristem Therapeutics announces that Japan’s Pharmaceuticals and Medical Devices Agency agreed with its methods for the manufacture of PLX-PAD cells.

CombiLac is a lactose-based, co-processed excipient, designed to ease oral solid dosage form development and manufacture in direct compression.

A vaccine patch may eliminate the need for traditional means of vaccine distribution, according to an article in NPR.

The VERISEQ nucleation technology offers a commercially viable technique for cryogenically generating a uniform dispersion of microscopic ice crystals (or ice-fog).

BASF announces that it will sell its custom synthesis business and parts of its API business to focus its expertise on pharmaceutical excipients.
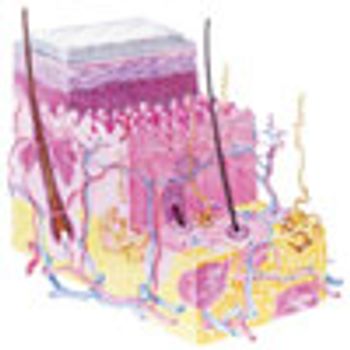
Advances in transdermal drug delivery, particularly with microneedles, are enabling a wider range of drugs to be delivered through the skin.

Bayer announces that it will pay up to $155 million, plus royalties, for the rights to Isis Pharma’s anti-clotting drug, ISIS-FXI.

FDA cites Yunnan Hande Bio-Tech for cGMP violations related to data collection and security.

An integrated pilot plant tests heteronucleation and continuous crystallization.
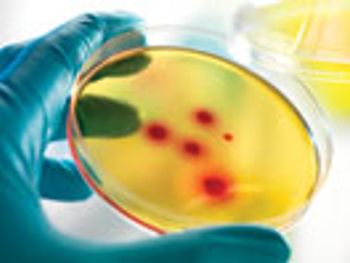
The author reports results of evaluations and concludes that a disinfectant composed of a low-concentration suspension of silver ions is completely sporicidal with only a one-minute contact time.
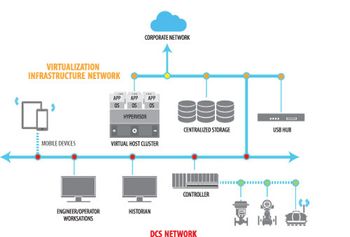
Virtualization has been mainstream in information technology (IT) for decades.
