Delivery systems that allow drugs to be administered as liquids, but to form gel within the eye, promise to improve efficacy and patient compliance.

Delivery systems that allow drugs to be administered as liquids, but to form gel within the eye, promise to improve efficacy and patient compliance.

The new facility expands the company’s commercial manufacturing capability at its Bend, Ore. site.

An ISPE guidance document, four years in the making, brings risk-based thinking, statistics, and Lean Six Sigma to cleaning validation.

Barry Holtz, Principal, at Holtz Biopharma Consulting, and Klyo Collaborative, spoke with Pharmaceutical Technology about collaborative success strategies for biopharm companies.

GSK will invest in an additional downstream isolation facility for amoxicillin production in Singapore.

The new column features Natrix’s signature macroporous hydrogel.
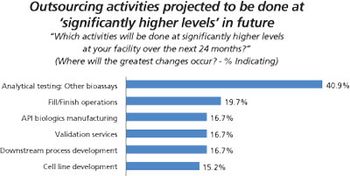
More biopharma companies choose outside service providers for assay testing.

Novasep's new antibody drug conjugate facility at its site in Le Mans, France will be commissioned in 2016.

BioSC Lab is the first in a line of next-generation chromatography equipment for protein purification in batch and continuous modes.

Reach Separations has announced plans to double its laboratory space at its Nottingham facility as a result of increased demand for its specialist purification services.

Monoclonal antibodies can facilitate the entry of radiopharmaceuticals into cells, David Scheinberg said at BIO 2015.

The International Conference on Harmonization finalizes Q&A document on APIs.

GSK announces it will invest $95 million to launch a US-based non-profit research institute, Altius, to research technologies and approaches in understanding gene control.

Presenters at IVT's Microbiology Week discussed best practices and recent guidance publications for microbial control in sterile and non-sterile pharmaceutical processes.

Experts at Eppendorf discuss common challenges in cell culture and share insights on possible solutions.

The University of Pennsylvania announces that it will collaborate with WuXi AppTech to research and develop gene vectors derived from recombinant viruses.

The agency creates initiative to stimulate pediatric drug development.

BPTF seeks changes in performance goals and fee payment schedule in GDUFA renegotiations.

The product, marketed as Sirdupla in the United Kingdom, is the generic version of GSK’s beta agonist and corticosteroid combination treatment for asthma.

The agency issues draft guidance on the development of drugs to treat Duchenne muscular dystrophy.

At this year’s ACHEMA, Malvern brings to the exhibition floor, its expertise and tools for measuring particle size and shape, zeta potential, molecular weight, and rheological properties for the characterization of dispersed systems.

Talks will explore key trends and issues in the pharmaceutical space and address the “latest industry buzz.”

Optima Pharma is exhibiting a range of technologies from sterile-filling and freeze-drying solutions to isolators and restricted access barrier systems.

Hüttlin GranuLean offers a compact solution for high-quality granulation processes.

Products on display include the newest addition to the Mobius line of single-use bioreactors; Cellvento CHO cell culture media for optimized production of specific cell lines; and new high-volume AFS water purification systems.