
Endolysin technology targets unwanted bacteria, including resistant strains

Endolysin technology targets unwanted bacteria, including resistant strains

The growing threat and spread of antimicrobial resistance continue to ring alarm bells worldwide.

At the request of FDA, US Marshals seize unapproved prescription drugs from Florida distributor.

Recipharm makes a strategic investment in Synthonics and partners in development of novel compounds.

The European Medicines Agency releases guidelines for addressing and reporting risks associated with medication errors.

As the prevalence of falsified medicines continues to increase, Switzerland is taking measures to secure its supply chain such as the implementation of serialization.

Aptar Pharma has partnered with Shanghai Sine Promod Pharmaceutical to develop and launch its new budesonide dry powder inhaler (DPI), which features Aptar Pharma’s user-friendly and cost-effective Twister DPI.

Quality-by-design tools improve efficiency in scale-up of pharmaceutical processes.
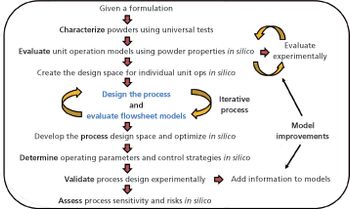
In-silico design facilitates process optimization and evaluation of process control strategies.

Drug developers understand the importance of early communication with regulators, but is EMA providing enough flexibility and support to companies?
Emerging controlled-release technologies could lead to more effective therapies in the near future.

Process design experimental data and risk assessments are used to predict expected process performance and establish process performance qualification acceptance criteria.

New legislation and changes in policy at FDA are leading to better control of the API supply chain.

The authors outline a validation approach for the manufacture of a “legacy product,” taking into consideration recent guidelines. The aim is to demonstrate the validity and capacity of the manufacturing process following a change in the manufacturer.

Multivariate data analysis (MVDA) is being used to effectively handle complex datasets generated by process analytical technology (PAT) in biopharmaceutical process development and manufacturing.

A consensus-based standard issued by NSF International incorporates regulatory and industry requirements into a single standard for the manufacturing and distribution of pharmaceutical excipients.

The White House confirmed that it would release a plan to tackle the growing problem of antibiotic-resistant superbugs.

Bristol-Myers Squibb acquired an exclusive license for Novo Nordisk’s discovery biologics research program.

Use of modeling software can help improve process understanding, and can be used in open- or closed-loop control.

Emergent BioSolutions announced FDA approval of Anthrasil, an inhalable treatment that targets Anthrax toxins, as well as a contract with BARDA to develop NuThrax, an anthrax vaccine candidate.

Clinical trial results suggest that monoclonal antibodies targeting the function of proinflammatory cytokine IL-17A in psoriasis may be significantly superior to other treatments.

Real-time, noncontact imaging and spectroscopy techniques provide insight into pharmaceutical processes.
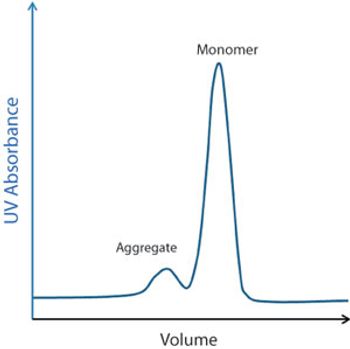
Several chromatographic resins are available for downstream purification.

Sharon Johnson, senior vice president, Global Quality & Regulatory Affairs, will head the organization.

NSF's consensus-based standard incorporates multiple regulatory and industry requirements into a single rigorous standard for the manufacturing and distribution of pharmaceutical excipients.