The parenteral manufacturing industry is taking action to address particulate contamination issues.

The parenteral manufacturing industry is taking action to address particulate contamination issues.

Measuring syringe plunger force using a texture analyzer instrument.
This review highlights relevant physicochemical drug properties and formulation design considerations critical to quality and performance of the sublingual tablets.

Bristol-Myers Squibb announced that they have reached an agreement with F-star Alpha, giving BMS the exclusive option to acquire F-star's HER2-targeted breast and gastric cancer treatment.

Pharmaceutical Technology Europe spoke with Piero Iamartino, R&D director at Micro-Macinazione, about the role of micronization in pharmaceutical manufacturing.
Safer fluorinating reagents and access to GMP fluorination capabilities remain challenges in API synthesis.

With a quality-by-design approach, robust processes can help deliver quality product consistently.

Formulation will utilize Catalent?s OSDrC OptiDose multicore drug-delivery platform.

DuPont Nutrition & Health has added a new pharmaceutical excipient to the portfolio of ingredients for CPhI Worldwide 2014, in Paris this October.

Understanding how to identify, remediate, and prevent facility infection is crucial for product quality.

Molecular Profiles has expanded its capsule filling capability following significant investment into new equipment at its clinical manufacturing site in the United Kingdom.

Conventional tablets may no longer be the go-to solution.
A survey of the recent literature reveals numerous advances in asymmetric chemocatalysis.
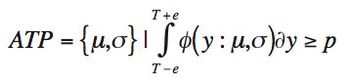
The quality-by-design principles used to control process variability are equally important to measurement systems because process variability includes contributions from measurement system variability. The authors use real-life examples from drug development projects to outline how an understanding of chromatographic measurement system variability might be achieved.

With a quality-by-design approach, robust processes consistently can help deliver quality product.
Advances in engineered particles and the subsequent reduction in the API mass required to achieve a therapeutic dose can lead to a reduction in side effects.
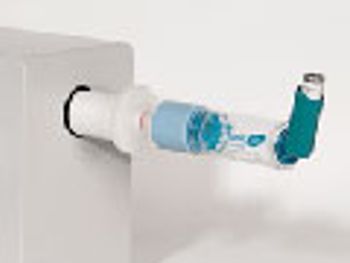
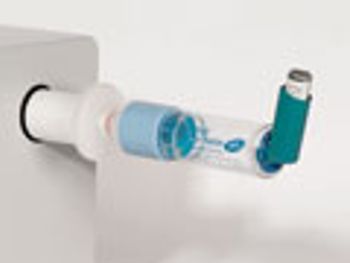
The role of add-on devices and how they affect drug delivery with a pressurized metered dose inhaler.

Changing regulations are impacting the identification and monitoring of variable materials in excipients.
New excipients and improvements to existing excipients are needed to facilitate access to new drugs for patients.

The quality and composition of excipients can vary due to environmental factors, processing methods, raw material quality, manufacturing location changes, and even operator actions.

Using a model quality risk-management process according to ICH Q9, the authors discuss ways to apply this guideline.

API development and manufacturing companies expanded their capabilities, built partnerships, and achieved milestones in regulatory inspections.

Customers are looking to reduce risk, increase performance, and optimize productivity.

Annual study shows CMO technical expertise is not enough.

Shrinking facility size, growth of biologics, and emerging market demand influence pharma investments.