
Single-use components aid efficiency in automated personalized therapy manufacturing.

Single-use components aid efficiency in automated personalized therapy manufacturing.

Sartorius Stedim Biotech’s BIOSTAT A is a compact bioreactor and fermenter with accessible peristaltic pumps, probe ports, and supply connections.

Dual sourcing is one of many possible solutions to securing the supply chain.

Recent technology introductions demonstrate that accuracy, efficiency, and usability are top of mind for pharmaceutical industry professionals for analytical instruments.

Biotage has launched a purification technology, Accelerated Chromatographic Isolation (ACI), which converts simple flash purification into a faster and more economical way to isolate pure compounds.

New advanced aseptic manufacturing technologies are available for filling liquid pharmaceuticals, including biologics.

Innovative polyethylene film sets new benchmark

Bioreactor for Mammalian Cell Culture

What challenges do biopharmaceutical manufacturers face when deciding to move from a fed batch process to a continuous process?

Application of single-use technology in a parenteral facility for syringe filling.

Catalent's Advasept platform uses blow-fill-seal technology to aseptically manufacture, fill, and seal a polymeric primary container for injectable drugs.

Both upstream and downstream processes can benefit from continuous manufacturing advantages.

Higher antibody titers and a growing demand for smaller-volume, flexible processes are creating the need for more cost-effective downstream processing.
Automated sample handling, advanced glycan analysis, and specially designed columns are help speed up confirmation of the biosimilarity.

Consider these best practices when deciding to implement single-use components.

Data from BioPlan Associates’ 10th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production suggest that the interest in disposable devices has begun to extend to biopharma operations beyond basic single-use bags and connectors.

After realizing benefits from the adoption of a single-use filling line for one specific product, Patheon took the leap and installed a single-use filling line in a new facility.

FDA updates guidance to reflect advances in technology.

RABS is a flexible barrier system that maximizes product control but minimizes operator interaction when best practices are followed.

PIERRE FABRE MEDICAMENT PRODUCTION has 22 years experience in isolator technology for aseptic filling of high potent freeze-dried injectable products.

Shimadzu's LCMS-8040 combines newly improved ion optics and collision cell technology with proprietary ultrafast technologies.
There is no harmonized guidance on pre-use integrity testing of sterilizing filters, prompting discussion among users as to whether such testing is necessary.

Recycling is becoming a viable option for disposal of single-use systems.
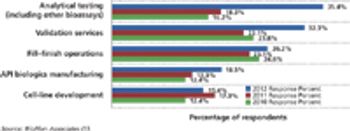
Budgets for biopharmaceutical activities are gaining in select functional areas except outsourcing.

Industry experts share perspectives on analytical instrumentation, methods, and data analysis.