Industry experts discuss formulation and technical challenges in multilayer tablet manufacture.

Industry experts discuss formulation and technical challenges in multilayer tablet manufacture.

Nanosized systems are important in drug delivery. Such nanosized systems include liposomes, nanocrystals, micelles, colloidal particles, quantum dots, and dendrimers. Dendrimers are class of synthetic macromolecules with highly branched, monodispersed, circular, and symmetrical architecture that are used as carrier molecules in drug delivery.

The author describes how providing appropriate information about the API in the Common Technical Document can aid FDA's review of an abbreviated new drug application.

New product reviews for January 2012.

Pfizer and GlaxoSmithKline have announced separate agreements with the GAVI Alliance to supply pneumococcal vaccines to developing countries. Pneumococcal disease can lead to pneumonia, meningitis, and sepsis, and is one of leading causes of death in children under the age of five in developing countries.

AstraZeneca Acquires Chinese Generic-Drug Company; Takeda Makes Management Changes; and More.

Pfizer and GlaxoSmithKline have announced separate agreements with the GAVI Alliance to supply pneumococcal vaccines to developing countries.

Last week, the US Department of Health and Human Services and Novartis Vaccines and Diagnostics dedicated a manufacturing plant that can create influenza vaccine using cultured animal cells instead of the conventional expression system of fertilized eggs.

Samsung and Biogen Idec agreed to invest $300 million to establish a joint venture to develop, manufacture, and market biosimilars. The deal is Samsung's latest effort to strengthen its position in biosimilars.

On Dec. 6, 2011, FDA announced that a public meeting will be held on Dec. 16, 2011 to discuss recommendations for a user fee program for biosimilar biological products for fiscal years 2013–2017.
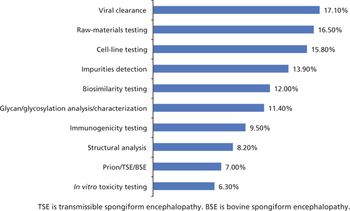
Biosimilar manufacturers need better expression systems and analytical tools to compete.
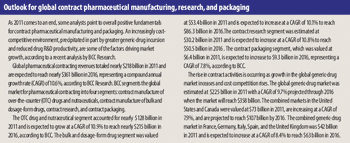
Expansion activity was limited as fine-chemical producers and CMOs of API and intermediates grapple with changing industry fundamentals.

As the excipient supply chain becomes more complex, industry must up the ante to comply with new standards and regulations.

New product reviews for December 2011, focusing on manufacturing.

Drug shortages, supply-chain security, generic-drug incursion, and flexible manufacturing models are some of the issues shaping the bio/pharma industry.

Precedents set in the historic Barr case continue to raise questions over suitable sample-size criteria.

A Q&A with Deborah Tanner, executive vice-president and group president of R&D laboratories at Covance, on recent industry trends.

The authors developed a metronidazole-based floating drug-delivery system to investigate the effect of rate-controlling polymers on release pattern and duration of buoyancy in matrix tablets.

Readers react to the economic turmoil of the past year and look longingly forward to 2012.

Pharma companies must balance demand for new drugs while facing reduced R&D spending.

On Sept. 27, 2011, FDA sent Genentech a Form 483 listing several violations at the company's South San Francisco, California, plant. The violations included problems with investigations into batch failures, inappropriate equipment design, and insufficient protection against contamination. FDA visited the plant, which produces the cancer drug Avastin, 13 times in September 2011 and made four observations.

Small drug companies with hopes of achieving $1 billion in sales can pursue various strategies.
Pharmaceutical companies are responding to the high cost of introducing new drugs to market in different ways.

Researchers develop various catalytic approaches for improving yield, purity, stereoselectivity, and process conditions.

Novartis AG and Novartis Pharmaceuticals Corporation have been focusing research efforts on rare diseases since the company was established in 1996.