
Congressional hearings were held last week on the Food and Drug Administration Globalization Act discussion draft.

Congressional hearings were held last week on the Food and Drug Administration Globalization Act discussion draft.

Also, FDA removes OAI status for Watson's Florida facility, executive management changes as GSK, more...

Polyplus-transfection, a company that researches, develops, and commercializes drug-delivery solutions for biomolecules, created a new technology designed to enhance in vivo delivery of small interfering RNAs (siRNAs) when they are associated with a cationic polymer.

To keep pace with proposed increases in the number of manufacturing inspections, the US Food and Drug Administration's Office of Regulatory Affairs (ORA) has announced it will increase laboratory capacity and staff.

Merck and Company?s West Point, Pennsylvania facility received a Warning Letter from the US Food and Drug Administration.
Scientists are giving up on a preventive vaccine for AIDS, but there are lessons to be learned.

With counterfeiting on the rise and Europeans worried their backyard is becoming a base for such illegal activity, legislators have proposed a series of solutions that span the continent and abroad.

Getting IT, engineering, and manufacturing on the same page requires a delicate balance.

Enterprise process control and management (EPCAM) is a new strategy for healthcare manufacturers based on recent process-control breakthroughs in the electronics industry.

ISA 100.11a and WirelessHART both seek to become the global standard for industrial wireless automation.

An authoritative book helps drug developers face one of their toughest problems.

The US Food and Drug Administration announced its Pharmaceutical GMPs for the 21st Century initiative six years ago. This article reports on the outcome of a recent workshop on this topic and the action plan set forth.

The less complex nature of excipient manufacturers, as compared with API manufactures, carries many benefits.

Editors' Picks of Pharmaceutical Science & Technology Innovations
Brief pharmaceutical news items for May 2008.

Regulators face demands to improve postmarket surveillance and meet review deadlines.

INTERPHEX 2008 offered visitors novel experiences and many stimulating sessions.

WFI system deficiencies and damp tax records cause problems for two plants

According to the latest figures from IMS Health, Inc., Japan's pharmaceutical market is expected to grow 1–2% this year compared to global industry growth expectations of 5–6%.

Recent advances in SEM, particularly the incorporation of automation and software, have made simpler, lower-end SEM instruments easy to operate and have improved the capabilities of larger, sophisticated instruments.

The current trend within the pharmaceutical industry toward more efficient development, manufacturing, and specification is fueling demand for analytical tools that provide highly relevant information. Effective powder characterization has a valuable role to play.

Paul Sheskey of Dow Chemical provides an update on foam granulation technology.
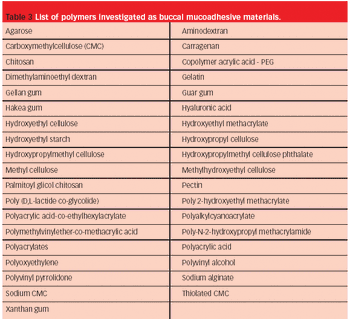
The adequate absorption and transport of drugs in the body is part of optimal therapy. Drug administration perorally is easy, common and traditional, but occasionally alternative routes are required.
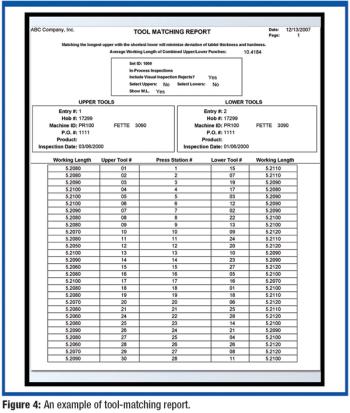
Inspecting punches and dies can be time-consuming and costly for tablet manufacturers. Advances in technology, however, have greatly improved in-process inspections. The author examines improvements in equipment and computer software for in-process tool inspections.