
Pre-Interphex Showcase: Cleanrooms
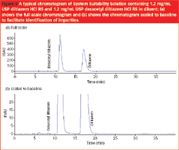

Pre-Interphex Showcase: Cleanrooms

Also, Genmab will acquire PDL Pharma's Minnesota manufacturing facility, DSM Pharmaceuticals appointed Hans Engels president and business unit director, more...

The US Patent and Trademark Office rejected the patentability of claims of a patent by Genentech that related to certain methods used to make antibodies and antibody fragments by recombinant DNA.

APP Pharmaceuticals will boost manufacturing of therapeutic multidose vials of heparin.

The US Food and Drug Administration has issued generics manufacturer Vintage Pharmaceuticals a warning letter stemming from July through August 2007 that found observations pertaining to microbial contamination and failures in testing and documentation procedures.

The US Food and Drug Administration recently revealed that the active ingredient used in the production of Baxter International Inc.'s recalled drug Heparin was made in a plant in China.

Also, Biocon will acquire 70% of AxiCorp, ARIAD Pharmaceuticals promoted Richard W. Pascoe to the new position of COO, more...

The US Food and Drug Administration issued a draft guidance for industry titled "Good Reprint Practices" regarding the distribution of medical or scientific journal articles and reference publications that involve unapproved uses of FDA-approved drugs and medical devices.

Preparing sterile products requires manufacturers to control microbial quality. Sterility and endotoxin content are critical because failure to properly manage them can seriously harm, or even kill, patients.

To ensure an effective treatment, a patient often must take equal doses of an active pharmaceutical ingredient (API) at regular intervals.

Featured products from this issue of Equipment & Processing Report

Baxter Healthcare Corporation is providing an update to its January 2008 heparin sodium injection 1000 units/mL 10 and 30mL multi-dose vial voluntary recall of nine lots, which the company initiated as a precautionary measure due to an increase in reports of adverse reactions that may be associated with the drug.

Scientists at The Wistar Institute are taking further steps into understanding a gene regulator that can lead them closer to developing new cancer therapies.

Chesapeake to relocate and expand, AVI BioPharma appoints CEO, More...

Nektar Therapeutics eliminated approximately 150 positions as part of a restructuring program designed to help the company complete its transition from a drug-delivery service provider to a drug-development organization.

Amira and GSK Form Agreement, Xenome Appoints Ian Nisbet CEO, More...

Amgen will partner with R&D firm Takeda Pharmaceutical Company Ltd. toward the development and commercialization of 13 of Amgen?s molecules, with one included as an option.

The US Food and Drug Administration posted on its website a Jan. 24 Warning Letter to Novartis Vaccines and Diagnostics.

Contract manufacturers expand capabilities in aseptic processing, clinical-trial materials supply, and cytotoxic manufacturing.
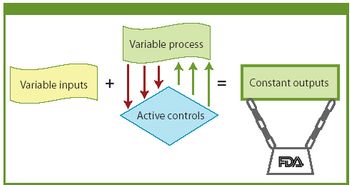
FDA is modernizing and streamlining current good manufacturing practices. The author examines FDA's evolving approach to quality systems and how a manufacturer can implement a quality system framework.

The rise in overseas manufacturing undermines FDA oversight of drug quality.

PharmTech's polls feature user feedback on issues facing the pharmaceutical industry.

Brief pharmaceutical news items for February 2008.

A news roundup for February 2008.

The author suggests a route for nanotechnology's future in the pharmaceutical industry.