
Microbix Biosystems has established a team of experts to help manufacturers boost their production of influenza vaccine.

Microbix Biosystems has established a team of experts to help manufacturers boost their production of influenza vaccine.

FDA approvals renew the threat of competition from India and China.

FDA's long-awaited GMPs for supplements appear as food and drug safety concerns override lingering opposition.

By vetoing stem cell research funding, the President is vetoing potentially life-saving treatments.

Makers of temperature-sensitive products constantly seek to ensure proper conditions during shipping and storage.
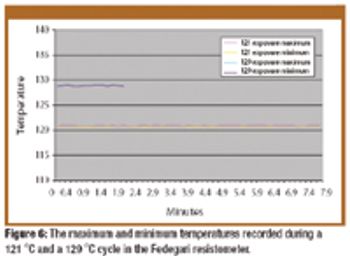
The authors examine advances in the design and the application of biological indicator evaluator resistometer vessels used to measure the resistance of bacterial spores in monitoring sterilization processes.

Production sometimes follows the law of supply and reprimand.

Cook, ABB, Tyco, and Mettler-Toledo Gain New Managment
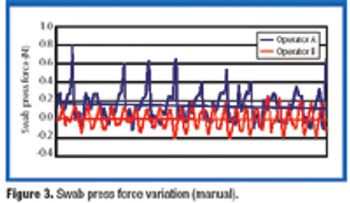
This article presents a study of an aseptic environmental monitoring system for surface contamination at critical areas using a robot.

Industry and regulatory experts provide advice on inspection preparation and best practices.
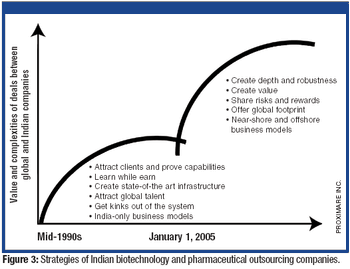
The tightening of intellectual property rights in India under GATT/TRIPS was a crucial inflection point for pharmaceutical outsourcing in India.

Risk management is essential in any successful outsourcing partnership. The author outlines the steps toward identifying, understanding, and controlling risk in key manufacturing areas.
The authors analyze the supply–demand trends for contract biologics manufacturing and the strategies of pharmaceutical and biotechnology companies and their suppliers in the value chain.

This article is written to assist clinical manufacturing representatives at pharmaceutical companies who are faced for the first time with outsourcing the manufacture of clinical supplies. The author describes the identification, writing, and execution of documents required to support the contract manufacture of products for clinical studies.
Infrastructure must be in place before jumping into outsourcing.
Using basic project management tools and ideas in the transfer process saves time and money and ensures high success rates.
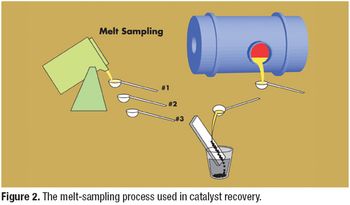
Catalysts are important tools in the synthesis of active pharmaceutical ingredients. Recovery of precious-metal catalysts from a pharmaceutical manufacturing process is a factor in cost control and environmental compliance.

The company begins production at a new $100-million manufacturing facility for prefilled injection systems, plans further investment in packaging facilities, and targets both early-phase development and commercial manufacture.
Catalytic routes to producing atorvastatin and sitagliptin are recent advancements.

Merck & Co. acquires NovaCardia, Sanofi Pasteur completes vaccine-manufacturing facility, more

An industry survey finds that pharma's outsourcing practices keep the industry from reaping the full benefits of outsourcing.

Novartis Vaccines plans to produce approximately 40 million doses of its ?Fluvirin? vaccine for distribution in the United States during the 2007?2008 flu season.

The European Medicines Agency?s (EMEA) Committee for Medicinal Products for Human Use (CHMP) adopted 11 positive opinions, 3 of which are tied to similar biological medicinal products.

More than five million US adults import prescription drugs from other countries, according to a survey conducted by the Pharmaceutical Research and Manufacturers of America.

Novartis Vaccines and Diagnostics, a division of Novartis AG (Basel, Switzerland) and IntercellAG (Vienna, Austria), a biotechnology company, will collaborate toward developing a broad range of vaccines.