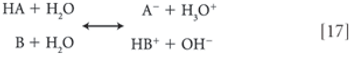
Through consideration of the ionic equilibria of acids and bases, one may readily calculate the formation constant of a salt species solely on the basis of knowledge of the pKA value of the acid and the pKB value of the base.

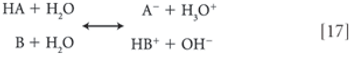
Through consideration of the ionic equilibria of acids and bases, one may readily calculate the formation constant of a salt species solely on the basis of knowledge of the pKA value of the acid and the pKB value of the base.

Expanded access to drugs for seniors has increased demand and focused attention on costs.
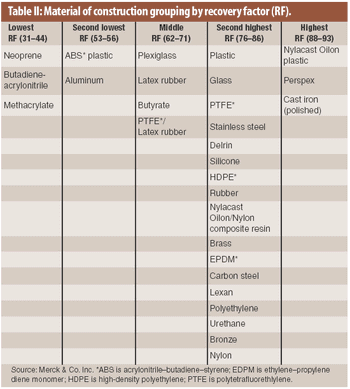
The material of construction is a factor in the recovery of residue in cleaning validation. An analysis of existing recovery data showed that recovery factors for drug products on various materials of construction may be categorized into several groupings.
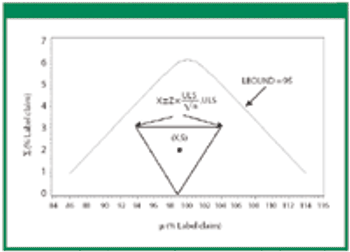
Revisions to the United States Pharmacopeia's (USP) uniformity test require manufacturers to establish new acceptance limits. The authors present their method for calculating acceptance limits consistent with USP's revised content-uniformity test requirements.
Security, the environment, ageing populations, the bio-boom and cost control are just a few of the drivers that will influence pharmaceutical packaging for the remainder of this decade.
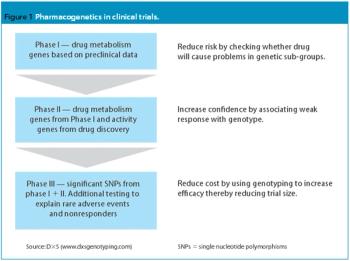
The completion of the Human Genome Project in 2003 led to a flurry of predictions regarding the application of pharmacogenomics to drug development. With US and European regulatory authorities finally on the verge of issuing guidance on the use of pharmacogenomics, drug development is all set to change.

Last year, a monoclonal antibody, TGN1412, led to potentially fatal adverse effects in a small group of Phase I volunteers in London. In the wake of this incident, EMEA has drawn up new guidelines that could lead to demands for more data on novel biologics. They may have implications for both manufacturing and clinical trials of biopharmaceuticals that are considered to pose a high risk to patients.

The UK, with its high quality educational capabilities in bioscience, has an opportunity to become the global hub of bioscience training, but must act quickly to secure this position.
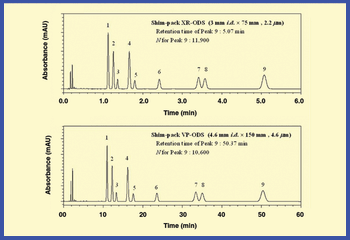
The authors review methods for reducing analysis time and increasing throughput that are reliable and maintain data integrity.
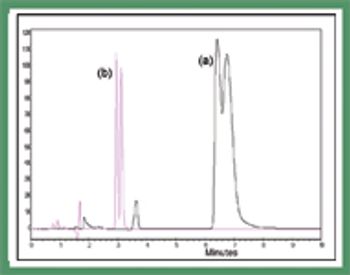
Polysaccharide-based chiral stationary phases have been developed that comprise chiral selectors immobilized on their support rather than being physically coated. These materials are completely solvent stable, thereby increasing selectivity and and enabling the development of new chiral selectors that have been too unstable in a coated form for general use.

The combination of supercritical fluid chromatography with chiral separation media offers several analytival advantages over traditional liquid chromatography techniques.

The scale-up of manufacturing processes to clinical production can be complicated and expensive, with many issues to consider. This article describes some of the common and less obvious pitfalls encountered by biopharmaceutical companies when scaling up protein production processes, and how to avoid them.
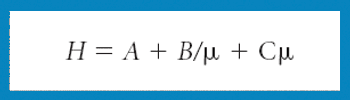
Ultrahigh pressure liquid chromatography maximizes efficiency, but, as defined by the resolution equation, the stationary phase is still a crucial consideration when attempting to resolve mixtures of compounds.

High performance liquid chromatography has become an efficient technique at the production scale, and simulated moving bed chromatography provides several benefits during processing.
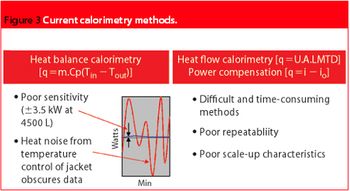
To date, calorimetry has not been given a fair trial in the PAT arena. Recent advances in reactor technology and design will ensure that real-time calorimetry is the present and future of PAT.
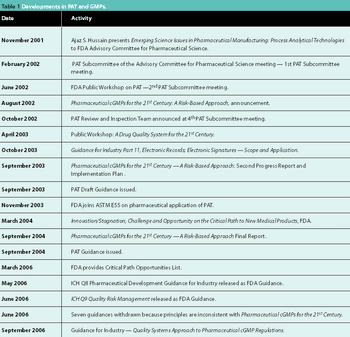
Recent regulatory initiatives have emphasized the need to improve pharmaceutical manufacturing. PAT marked the beginning of a number of regulatory efforts to encourage innovation and a transition towards science-based manufacturing. This article reviews the progress of the regulatory initiatives and describes two significant research initiatives to develop a future pharmaceutical manufacturing environment based on scientific understanding of pharmaceutical materials and processes.

Company and People Notes: Evotec and Renovis enter agreement, Amgen to lay off 675 workers, more.

The European Medicines Agency?s Committee for Medicinal Products for Human Use recommended the lifting of the suspension of the marketing authorization for ?Viracept? (nelfinavir mesylate) from Roche and the re-introduction of the drug in the European Union.

Reflecting a strategic interest to strengthen its position in biologics, Bristol-Myers Squibb agreed to acquire the biopharmaceutical company Adnexus Therapeutics for $430 million.

The US Food and Drug Administration has denied shipments of active pharmaceutical ingredients manufactured at a production facility of Kunshan Chemical and Pharmaceutical Co. for violation of good manufacturing practices, according to an FDA warning letter issued Sept. 6, 2007.

Company and People Notes: Catalent expands Bolton, UK, warehouse; Biotica appoints Edward E. Hodgkin as CEO and director; more.

Pfizer plans to cease all remaining manufacturing operations at its facility in Sandwich, Kent, United Kingdom. The closure will result in the loss of approximately 420 jobs, phased over the next two years, according to a company release

Bayer Schering Pharma officially completed the acquisition of Novartis?s biologics manufacturing facility in Emeryville, California.

The European Commission (EC) has deemed research-performing small- and medium-sized enterprises (SMEs) ?the entrepreneurial stars of Europe?, hoping to raise ?800 million extra funds for them.

Sartorius has officially started up operations at its new plant in Beijing. By building the new facility, the company has now doubled its production capacity in China from approximately 4000 m2 to more than 8000 m2; an additional building phase makes it possible to even quadruple the floor space in the future. The investment volume for the new plant site is about ?10 million.