
Company and People Notes: BASF raises prices on excipients; Verus Pharmaceuticals appoints president and CEO; more.

Company and People Notes: BASF raises prices on excipients; Verus Pharmaceuticals appoints president and CEO; more.

Numerous healthcare companies feature among the top 20 of the world?s largest corporate R&D spenders.

Submitting a safety update to European regulators, Novartis provided data showing its 100-mg once-daily dose of ?Galvus? (vidagliptin), an oral type 2 diabetes treatment, has more frequent liver enzyme elevations in patients than its already approved 50-mg once- and twice-daily doses.

Pfizer's Lipitor patent revoked in Germany, promotions at Charles River Labs, more.

Wyeth Consumer Healthcare launched a voluntary recall and replacement program for US retail outlets that sell several ?Robitussin? and ?Children?s Dimetapp Cold and Chest Congestion? products.

US Food and Drug Administration Commissioner Andrew C. von Eschenbach testified before Congress last week to outline the agency’s inspection process for foreign drug manufacturers and efforts to improve the agency’s information technology systems.

The US Food and Drug Administration’s effectiveness in regulating the manufacture of pharmaceutical products and active pharmaceutical ingredients at foreign facilities was questioned at a Congressional hearing last week. Congress, industry, and government officials weighed in on the issue.

King Pharmaceuticals, and Acura Pharmaceuticals have entered into a license, development and commercialization agreement for the United States, Canada, and Mexico, encompassing a potentially wide range of opioid analgesic products utilizing Acura?s patented Aversion (abuse-deterrent) Technology platform.

Editors' Picks of Pharmaceutical Science & Technology Innovations
Information technology is the glue that should unify a company while ironically, it enables further fragmentation. Experts talk about the successes and challenges for IT in helping a company function efficiently.

Market demand for cytotoxic drugs is leading CMOs to expand their API manufacturing and formulation services.

The US Food and Drug Administration launched a new program on Oct. 4 to increase the number and variety of generic drugs available to the public, beginning in fiscal year 2008. The Generic Initiative for Value and Efficiency (GIVE) will use existing resources to help the agency "modernize and streamline the generic drug approval process," according to FDA.
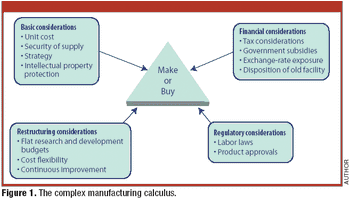
Candid comments from a Big Pharma executive highlight the complexity of contract manufacturing.

In this study, fault tree analysis applied to a water pretreatment and purification installation exposed cause-and-effect complex interrelations in possible fault events.
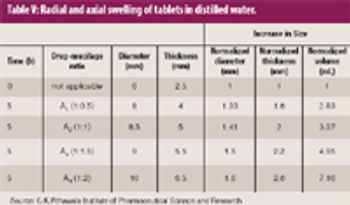
Natural gums and mucilage have been widely explored as pharmaceutical excipients. The goal of this study was to extract mucilage from the leaves of Aloe barbadensis Miller and to study its functionality as an excipient in pharmaceutical sustained-release tablet formulations.

News and Views

Thin are the lines that separate stability, statistics, and chaos.

A book illustrates the potential for nanoparticulate drug delivery, and how much about them remains to be understood.

New FDA act reshapes drug development and marketing to restore public trust in pharmaceutical regulation.

Mixed-flow impeller systems exhaust laboratory workstation fume hoods, prevent reentrainment into the facility and adjacent facilities, and help companies comply with appropriate pollution-control standards.

With appropriate standardization from an interfacing point of view, separation science will find a broader audience in PAT.
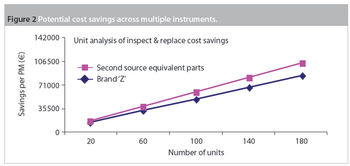
Maintenance and service-related items are often the second-largest budget element in a laboratory after salaries and benefits. Within maintenance, preventive maintenance (PM) is a substantial portion of the budget. Traditionally, PM was an equipment maintenance philosophy based on replacing, overhauling or remanufacturing a piece of equipment at fixed intervals, regardless of its condition at the time. In essence, it involved fixing something that wasn't necessarily broken and this approach is still widely used in the pharmaceutical industry.
Pharmaceutical manufacturers are under increasing pressure to shorten time-to-market, produce treatments with unpredictable product lifetimes, provide greater flexibility and, at the same time, comply with ever more stringent quality, validation, stability and traceability constraints. While this is encouraging for the contract manufacturing sector, it creates the need for even greater manufacturing flexibility.

Vaccines are needed against old and new infectious disease threats - polio and other childhood illnesses, bioterrorism and pandemic flu. They are also emerging for cancer immunotherapy and for treating addiction. While vaccines are among some of the most successful biotech products, their large-scale manufacture involves some special demands, such as maintaining a good working cell bank and gearing up for production on an 'as needed' basis.

The crystalline structure of pharmaceutical solids can sometimes be altered during processing. X-ray powder diffraction and near infrared spectroscopy can be used to determine the amorphous and crystalline content of a model substance. The two techniques' precision, accuracy, detection limit and the speed of analysis are compared.