
Aptuit expands its drug-development capabilities with the formation of Aptuit Laurus to take advantage of the growing pharmaceutical outsourcing market in India.

Aptuit expands its drug-development capabilities with the formation of Aptuit Laurus to take advantage of the growing pharmaceutical outsourcing market in India.

FDA must increase inspections of foreign API manufacturing facilities as more production moves offshore.

Contract manufacturers slim down to improve profitability.

Appendix: definitions and regulations, Federal Food, Drug, and Cosmetic Act; Appendix: definitions and regulations, Title 21 Code of Federal Regulations; Appendix: definitions and regulations, Compliance Policy Guides

News and Views

Going digital can produce high-quality, full-color labels at potentially lower cost.

User-fee legislation will require more testing and data disclosure to prevent unsafe drug use.
The influence of magnesium stearate (MgSt) on powder lubrication and finished solid-dose properties presents big challenges to drug manufacturers.

Jason Kamm, managing consultant with Tunnell Consulting discusses the challenges and opportunities for pharmaceutical manufacturers in ICH Q10, the draft guidance on pharmaceutical quality systems issued by the International Conference on Harmonization.


How the Indian pharmaceutical sector is reinventing itself

The draft guidance ICH Q10 for pharmaceutical quality systems is part of the ongoing move to a science- and risk-based approach in manufacturing.

In a nation of more than 1 billion people, the importance of vaccines goes beyond healthcare-it is a matter of national security. Armed with this belief and a philanthropic vision that most Indians could be protected against hepatitis, DT–Polio, and other afflictions, Dr. Varaprasad Reddy entered the nascent Indian biotechnology sector in 1992 and has since managed to threaten the monopoly of large laboratories. That year, the Hepatitis B vaccine cost $33 per shot, and yet some families were subsisting on less than $1 a day. Meanwhile, India was importing only 180,000 doses per year.

Useful Contacts

Poor processing and misguided projections lead to trashed product.

This article presents collaborative positions among excipient manufacturers, drug product manufacturers, and members of the US Pharmacopeia on key issues pertaining to the control of pharmaceutical excipients stemming from a recent Pharmaceutical Quality Research Institute workshop.

Larger and strategic sampling and testing plans can improve process understanding and characterization.

The recently published Orange Guide 2007 contains significant changes to the GMP requirement placed on pharmaceutical manufacturers, but there have been additional changes to good distribution practice that should not be overlooked.
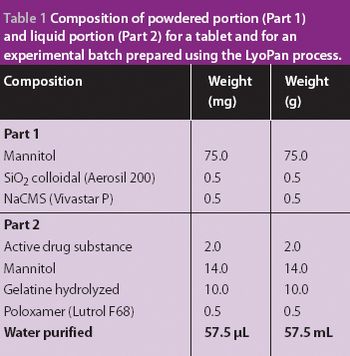
A new economical method for producing fast-melting lamina-like dosage forms.
In biomanufacturing, multiple sensors provide a wealth of data that could be used to enhance process understanding and assist in performance improvement. This article looks at how to move from a data-rich environment to one where the data are translated to useful information that leads to knowledge and, ultimately, process improvements.
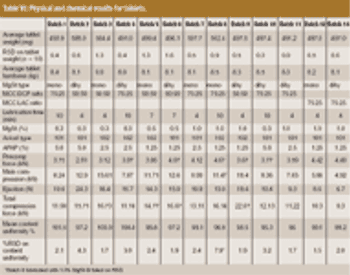
This article describes how rapidly disintegrating tablets containing a large quantity of an intensely bitter drug were successfully developed with a suitable level of masking, tablet hardness, disintegration property, dissolution profile and mouth feel.

Is consolidated distribution the key to combating counterfeit drugs and parallel imports?

Getting from a cell culture to a purified biotech product is a demanding exercise involving many operations. Increasing productivity in the upstream part of biotech production is placing new demands on the purification process, which may lead to adopting new technologies.

A new Raman spectroscopic method to detect magnesium stearate in powder blends and tablets is described. High-volume pharmaceutical manufacturing requires the use of lubricants to facilitate tablet ejection from compressing machines. However, lubricants may also bring a number of undesired problems that have been widely documented in pharmaceutical scientific literature. New analytical methods are needed to understand lubrication and provide process knowledge in support of FDA's process analytical technology initiative. The detection of magnesium stearate in lactose, mannitol, corn starch and other commercially important excipients is reported. The Raman spectroscopic method has a detection limit of about 0.1% (w/w) based on the 2848 cm-1 band that corresponds to the symmetric stretch of the methylene group in magnesium stearate.

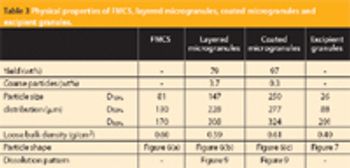
The authors evaluate the scalability of foam-granulation technology using continuous foam addition in high-shear granulation equipment at the laboratory, pilot and manufacturing scales. Immediate- and controlled-release model formulations were used. Continuous and batch addition of foam were compared for the controlled-release model formulation at the manufacturing scale, and physical testing was performed on the granules and finished tablets.