
NPIL Pharma, a subsidiary of Nicholas Piramal India Limited (Mumbai, India) has agreed to acquire the Morpeth, Northumberland, UK, manufacturing facility of Pfizer Inc. (New York, NY).

NPIL Pharma, a subsidiary of Nicholas Piramal India Limited (Mumbai, India) has agreed to acquire the Morpeth, Northumberland, UK, manufacturing facility of Pfizer Inc. (New York, NY).

GlaxoSmithKline PLC (GSK, London, UK) will invest more than GBP102 million ($188 million) over the next four years in a vaccine manufacturing plant in Singapore.

This week?s PharmTech Annual Event (www.pharmtechevent.com) in Somerset, New Jersey, targeted approaches to improving drug development and quality through optimizing processes, managing risk, and controlling variations in manufacturing operations.

The US Food and Drug Administration?s Counterfeit Drug Task Force (Rockville, MD, www.fda.gov) is recommending regulatory actions and the implementation of new technologies for reducing the risk of counterfeit drugs entering the United States. The group has followed up on its original 2004 report, in which it outlined the framework for protecting the public from counterfeit medicines, and an updated 2005 report with a third document encouraging electronic pedigrees, improved traceability in the drug supply chain, and the adoption of radio-frequency identification (RFID) tools.

The US Department of Health and Human Services (HHS, Washington, DC) awarded biotech company Cangene (Winnipeg, MB, Canada) a $362-million Project Bioshield supply contract for 200,000 doses of botulinum toxin immune globulin (heptavalent botulism antitoxin).

Recombinomics (Pittsburgh, PA) is again urging the World Health Organization to fully release all H5N1 avian influenza sequences, claiming their release would improve the selection of vaccines by helping scientists to identify the origin of the isolates and predict sequence changes.

Degussa AG (D?sseldorf, Germany) and Lynchem Co., Ltd. (Dalian, Liaoning Province, China) signed a contract to establish a custom manufacturing joint venture.

Gilead Sciences, Inc. (Foster City, CA) signed a definitive agreement to acquire the Canadian subsidiary Raylo Chemicals and most of its assets from Degussa AG (D?sseldorf, Germany) for 115.2 million euros ($147 million).

Noting several violations in current good manufacturing practice regulations, the US Food and Drug Administration has issued a Warning Letter to Wyeth Pharmaceuticals?s (Collegville, PA) manufacturing facility in Puerto Rico.

Sandoz (Holzkirchen, Germany), the generics arm of Novartis (Basel, Switzerland), received approval from the US Food and Drug Administration (Rockville, MD) for "Omnitrope" (somatropin [rDNA origin]), a follow-on version of a previously approved recombinant human growth hormone (rhGH) product.

Bristol-Myers Squibb Company (BMS, New York, NY) selected Devens, Massachusetts as the site for its new, large-scale, multiproduct bulk biologics facility.

China?s State Food and Drug Administration (SFDA, Beijing, China) issued a notice on May 18, 2006, requiring drug regulatory departments of provinces, autonomous regions, and municipalities to further strengthen the supervision and management of drug manufacturers.

Schering-Plough Corporation (Kenilworth, NJ) announced plans to phase out manufacturing operations at its Manati, Puerto Rico site, expecting to discontinue operations there by the end of 2006.

On May 26, AstraZeneca (Wilmington, DE) announced it would invest $100 million in research and development in China over the next three years.

I always suspected that our purchasing manager had agreed to this just to save money . . .
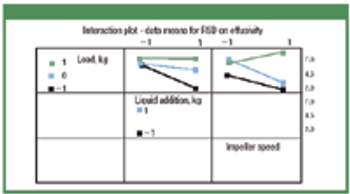
Thermal effusivity and power consumption may help predict granulation end point in high-shear granulators. In this study, power consumption was monitored and compared with percent relative standard deviation (RSD) on thermal effusivity measured at-line. Lactose monohydrate, microcrystalline cellulose, and magnesium oxide were granulated, and the effect of load size on granule growth in a fixed-volume granulator was evaluated using three load levels. Load size, liquid addition rate, and impeller speed were measured, and the correlation among RSD on effusivity, power consumption, mean granule specific surface area, and granule compressibility index were determined.

Predictable outcomes lead to greater manufacturing efficiency and speed time to value.

Interphex provided an opportunity to examine the latest pharmaceutical packaging concepts and packaging machines.
The biggest single recent trend in outsourcing solid-dosage processing has been the movement toward discovery and synthesis of more potent active pharmaceutical ingredients.
ORA leadership looks to staff redeployment and risk management to ensure product quality despite diminishing resources.
The construction of a new oral solid form (OSF) plant is an important decision and a real challenge. The team in charge of the basic conceptual design has to ensure that the new plant will be up-to-date and efficient not only at start-up, but for the next 15–20 years. This means that the project must be able to adjust to capacity changes, product changes and technology changes. It sometimes seems like an impossible challenge.
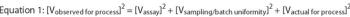
The first part of this article discussed general strategies for validation extensions to other test method components, laboratories and even different test methods.1 This second part provides practical tips on how to maintain test method suitability long after the formal completion of analytical method validation (AMV) studies.
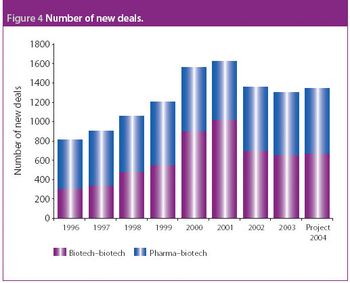
This column provides a summary of the key trends that are occurring within pharma–biotech alliances, as well as looking at biotech–biotech. It will explore the changing balance of power in pharma and biotech deals, providing examples and insights into which areas alliances are becoming increasingly popular.
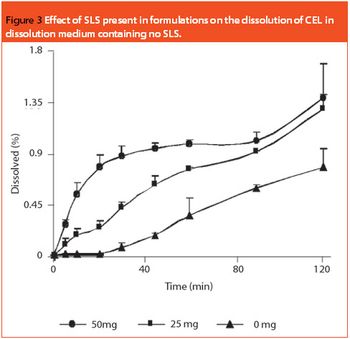
Bioequivalence with the reference product is the only reliable measure of demonstrating the therapeutic equivalence of a generic product to the innovator product. Systematic and comprehensive innovator product characterization can be used to make generic product development easier. This involves characterization of API and quantification of the critical excipients. The latter contributes towards performance of the final dosage form. This article describes the capsule formulation of a poorly water-soluble drug, celecoxib, which contains sodium lauryl sulphate as a critical excipient. The importance of a decoding process aimed at developing a generic product that matches the innovator formulation in a discriminating dissolution method is demonstrated.

Xethanol Corporation's (New York, NY) subsidiary CoastalXethanol signed a letter of intent with Pfizer Inc. (New York, NY) to purchase Pfizer's pharmaceutical manufacturing complex in Augusta, Georgia.