
How does the latest agency task force report resonate for pharma and radio-frequency identification?

How does the latest agency task force report resonate for pharma and radio-frequency identification?

Genzyme Corporation (Cambridge, MA) reports the US Food and Drug Administration (Rockville, MD) has approved the fill?finishing, packaging, and labeling of "Thymoglobulin" (antithymocyte globulin, rabbit) at its Waterford, Ireland facility. The approval allows Genzyme to begin manufacturing and distribution of Thymoglobulin from this facility.

The UK Medicines and Healthcare Products Regulatory Agency (MHRA, London) has reissued its recall of a specific batch of counterfeit ?Lipitor? 20-mg tablets. MHRA, in conjunction with Pfizer (New York City, NY), first issued the recall of batch number 004405K1 in July 2005. The new recall is in response to the discovery of more packages of the counterfeit drug in the United Kingdom.

Roxane Laboratories (Columbus, OH), a Boehringer Ingelheim company, is conducting a voluntary recall in the United States and Puerto Rico of a single manufacturing lot of "Azathioprine"tablets, USP 50 mg.

Seeking more than $5.8 million in damages and the recovery of nearly $1.8 billion in punitive damages, RxUSA Wholesale (Port Washington, NY) filed a complaint against 16 major US pharmaceutical manufacturers and 5 drug wholesalers.

Dietmar Hopp, cofounder of the German information technology giant SAP AG (Waldorff, Germany) is forming a new pharmaceutical company from the merger of two German biopharmaceutical companies: Axaron Bioscience AG (Heidelberg, Germany) and Lion Bioscience AG (Heidelberg, Germany).

Novartis (Basel, Switzerland) will build a cell culture-derived influenza vaccines manufacturing plant in Holly Springs, North Carolina. Construction is expected to begin in 2007.

In a move to strengthen its position in Western generic drug markets, Ranbaxy Laboratories Ltd. (Gurgaon, Haryana, India) acquired the Mundogen generic drug business of GlaxoSmithKline (GSK, London, England) in Spain, through Ranbaxy's Spanish subsidiary, Laboratorios Ranbaxy S.L.

A group of researchers from Georgia Institute of Technology (Atlanta, GA) are using high-throughput ionization techniques to identify and measure the ingredients in counterfeit drugs.

Although the number of anti-infective vaccines (as distinct from therapeutic vaccines for cancers and other noninfectious diseases) entering clinical study each year since 2000 has been higher on average than it was in the 1990s, this product area may see little additional growth through the rest of this decade, according to a recentanalysis from the Tufts Center for the Study of Drug Development (Boston, MA).

The Bayer Group (Leverkusen, Germany) plans to sell the diagnostics division of Bayer HealthCare to Siemens AG (Munich, Germany) for EUR 4.2 billion ($5.36 billion).

Vaccine maker Sanofi Pasteur, Inc. received a US Food and Drug Administration Warning Letter, dated June 30, citing "significant deviations" from current good manufacturing practices in the production of monovalent concentrates used in the company?s ?Fluzone? influenza vaccine.

Dutch biotechnology company Crucell NV (Leiden, Netherlands) and its technology partner DSM Biologics BV, a business unit of Royal DSM NV (Heerlen, Netherlands) will open a new research and development center that will specialize on further developing the "PER.C6" human cell line for the expression of recombinant pharmaceutical proteins.
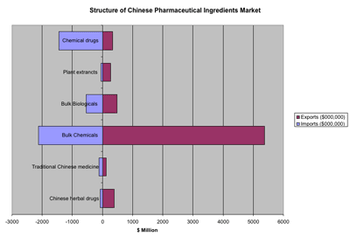
The sixth CPhI China exhibition, presented June 27?29 in Shanghai, offered a showcase for the explosive growth of the Chinese pharmaceutical sector.

MedImmune, Inc. (Gaitherburg, MD) reports that US Food and Drug Administration (Rockville, MD) has approved the company's supplemental biologics license application to use reverse genetics technology to construct new vaccine strains to produce seasonal influenza vaccines.

Biopharmaceutical company Lipoxen PLC (London, UK) has developed a Hepatitis E vaccine using its novel vaccine delivery technology "ImuXen," which the company claims to be easy to manufacture. According to the company, the proprietary liposomal formulation method delivers vaccine materials to the immune system in a manner designed to emulate the response of a natural encounter with the infection agent.
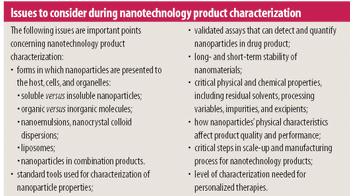
FDA is conducting laboratory research to understand better the ability of preclinical screening tests to identify potential risks and toxicities of nanotechnology-based drugs.

A cleverly developed patent portfolio can block or minimize competition long past a patent's 20-year lifespan.
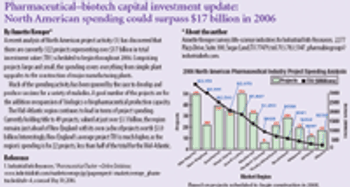
Pharmaceutical Technology provides perspective on pharmaceutical manufacturing activity, assessing the product portfolios, prescription volumes, revenues, and capital spending plans of more than 500 drug-makers. Novartis leads in diversity, with 889 products; Pfizer leads in number of scrips (352 million) and revenue ($44 billion). And generics companies are coming up on the inside.

This article summarizes changes to the Akers–Agalloco aseptic processing risk analysis model (first presented in Pharmaceutical Technology's November 2005 issue) as well as some of the underlying thinking behind the revision. The simplified model makes the method easier to use because of its greater flexibility of environmental control practice. It maintains the emphasis on human activity as the primary consideration in risk management for aseptic processing.

China is on the rise as a center for pharmaceutical R&D, but companies are still getting their footing for operating in China and the services industry has some maturing to do.

The hydrophilic matrix system continues to be the most popular and widely used strategy to achieve extended drug release. Hypromellose (hydroxypropylmethylcellulose [HPMC]) is typically the polymer of choice for the rate-controlling carrier in these systems.
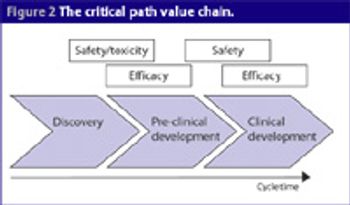
One of the biggest barriers research and academic institutions face is the ability to get discoveries made in the lab into clinical testing. Because only small amounts of drugs are used in these early studies, they represent fewer potential risks for people in these trials.
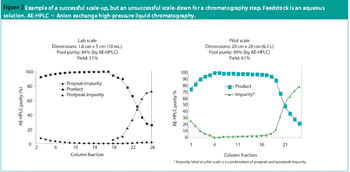
Constraints associated with equipment can make scale-down a challenging exercise.

The big challenge for pharmaceutical companies is to mathematically model their production processes, combining both data from the laboratory and the production process.