
The US Food and Drug Administration?s (Rockville, MD) Center for Drug Evaluation and Research (CDER) received 637 commercial investigational new drug (IND) applications in 2005, a 20-year high.

The US Food and Drug Administration?s (Rockville, MD) Center for Drug Evaluation and Research (CDER) received 637 commercial investigational new drug (IND) applications in 2005, a 20-year high.

Bringing Exubera to market requires extensive collaboration by Pfizer, Nektar Therapeutics, West Pharmaceutical Services Tech Group, and Bespak.

China's State Food and Drug Administration prepares to strengthen the enforcement of good manufacturing practices.

With single-enantiomer separations dominating the blockbuster charts, simulated moving bed chromatography and other multicolumn continuous chromatographic processes offer a quick route to clinical trial materials, along with the resolution, economy, and scalability to support tons-per-year production.

Manufacturing and formulation innovation spurs drug develop-ment, but raises new safety and quality issues.

With all the challenges that the manufacturing industry has had to deal with over the last ten years - growing compliance demands, increased competition and price pressures - it is perhaps not surprising that pharmaceutical firms are increasingly trying to streamline their manufacturing processes to maintain profit margins, speed up the time-to-market, as well as comply with market regulations that are becoming increasingly stringent.

The evolution of sophisticated monitoring systems has been accelerated because of stringent EU legislation surrounding the pharmaceutical industry and the escalating demand for preventative maintenance techniques. This article gives an overview of the benefits of implementing these systems in pharmaceutical manufacturing.
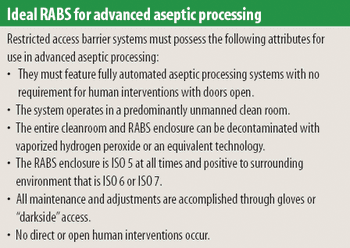
Any aseptic processing technology that allows intervention by gowned personnel during operation cannot be considered an advanced technology. Although a standardized definition of restricted access barrier systems has been developed, these systems fall well short of being classfied as advanced technologies.
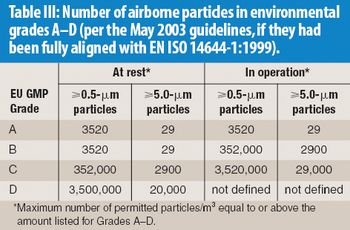
The author explores the importance of the proposals to revise Annex 1 of the EU GMPs in the context of the desire for science-based, internationally respected GMPs. Commentary also is provided about the relationship between this annex and CEN–ISO cleanroom standards.
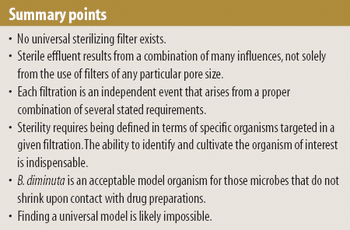
Model organisms are useful when validating sterile filtration, but successful retention of the model organism does not always guarantee that effluent is sterile. The authors explore the various factors that influence sterile filtration.
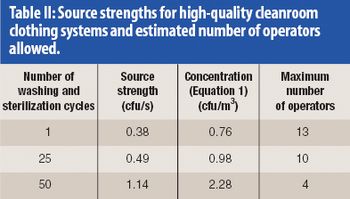
Calculations show the predicted contamination levels in cleanrooms with turbulent mixing air and with vertical unidirectional airflow when people are dressed in modern cleanroom clothing systems. Comparisons are made between operation theatres and cleanrooms.

The industry has acknowledged only recently the significance of the contamination risk posed by humans. The authors assert that this realization, together with technological advances, will lead to the elimination of human intervention and, hence, improved sterility.

There are few, if any, valid reasons not to install an isolator in a new aseptic processing facility.

The biotechnology company Discovery Laboratories Inc. (Warrington, PA, www.discoverylabs.com) reports that analysis of ongoing stability data from "Surfaxin" process validation batches indicates that certain stability parameters have not been achieved, and additional process validation batches will likely have to be produced.

Agilent Technologies Inc. (Palo Alto, CA, www.agilent.com) announced April 17 that it acquired SynPro Corp. (Boulder, CO), a contract manufacturer of oligonucleotide active pharmaceutical ingredients. Earlier, Dalton Pharma Services (Toronto), announced that it had completed a multi-gram CGMP oligonucleotide production facility.

Novartis (Basel, Switzerland) has closed on its $5.4-billion acquisition of Chiron Corporation (Emeryville, CA), paving the way for the creation of a new Novartis division focusing on vaccines and diagnostics.

Reverse Genetics Avian Influenza Vaccine Approved in France

GSK Begins Clinical Trials of Two H5N1 Vaccines
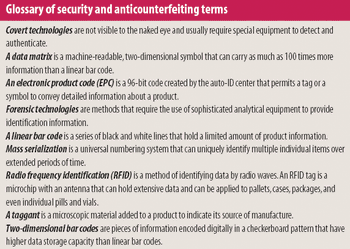
The need to curb drug counterfeiting is spurring development of track-and-trace and product authentication technologies.

Performing D-value and population verification is critical in the acceptance criteria for allowing a new lot of biological indicators into a facility before acceptance and use of the lot in validation work or routine monitoring of sterilization cycles.

Despite worries that industry is slow to adopt anticounterfeiting technologies, the 2006 Interphex program is rife with new methods for securing the supply chain.
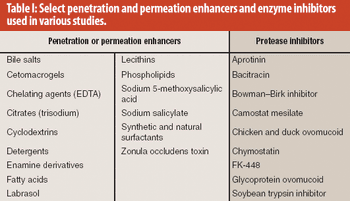
Absorption promoters and enhancers and enzyme inhibitors, either alone or in combination, can play an important role in improving the bioavailability of oral insulin.

Senior management must be the champions of change in companies that are struggling financially or organizationally.
Cell-culture technology and financial incentives give influenza vaccine makers a much-needed shot in the arm, but many downstream processing issues remain unaddressed.

The right choice [of coding and marking technology] depends upon the company's top priorities regarding legibility, cost, speed, ease of use, cleanliness and security.