
Chiron Withdraws Measles Vaccine

Chiron Withdraws Measles Vaccine

Development Begins on Mutated H5N1 Virus Vaccine

Proposed Legislation to Help Fight Counterfeit Drugs

Novavax, Bharat Biotech Team for Pandemic Influenza Vaccine Development

Facing still-sluggish market conditions and a changing world order in fine chemicals, the large Western custom manufacturers are responding by building their toolboxes in specialized technologies in chiral chemistry, catalysis, and biosciences and by adjusting their manufacturing networks via streamlining or investment in Asia.

Adding a cleaning step to the field-testing protocol, and combining it with the data generated to register sanitizing and disinfectant agents under FIFRA and the CEN TC 216 work program, produces a sanitation-and-disinfection validation methodology that is cost-effective, simple, and time-saving.
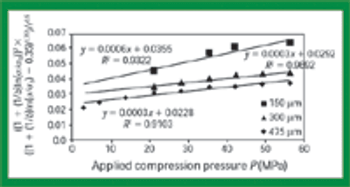
Quantitative data from the literature show strong relationships among average particle size, powder densification, tensile strength, and hardness.
Analytical tools and methods that require less water and detergent are gaining interest for faster, more efficient cleaning.

The authors encourage the investigation into whether the occurrence of grow-through and the diminution in the size of certain organisms when in contact with given liquids are the same phenomenon manifested under different circumstances.

PDA's Technical Report No. 39 provides guidance for protecting temperature-sensitive products.

A new FDA policy emphasizes quality control over GMP compliance in producing clinical trial supplies.
Because of the growing popularity of single-use materials, the identification, characterization, and qualification of new materials used for disposable processes have become increasingly important for both regulatory and production purposes. This article describes one approach to identifying and validating the materials used in a disposable filling process.

Secondary sensors help protect feedback control systems from the effects of sensor drift.

GSK Gains Approval for Rotavirus Vaccine in Infants; FDA Review Extended for Merck’s Shingles Vaccine

The PAT guidance indicates a variety of risk-based approaches to managing the introduction of on-line analysers into existing processes with the aim of minimizing the regulatory burden for the manufacturer and encouraging innovation.

Clean rooms are areas in which it is essential that microorganisms are not allowed to proliferate because they could contaminate pharmaceuticals and directly affect human health.

Pharma companies are forming alliances with less established biotech companies, which significantly drive the technological innovation and the overall market growth.

FDA Recommends Changes for 2006-2007 Flu Vaccine

Baxter Wins Contract to Develop Cell-Based H5N1 Vaccine

Applikon Biotechnology

Cambrex (East Rutherford, NJ) to produce Geron (Menlo Park, CA) telomerase anti-cancer vaccine. Generex (Toronto, ON.) files IND for synthetic vaccine to stimulate cell-mediated immunity to avian influenza. Dow Agrosciences (Indianapolis, IN) wins USDA approval for veterinary vaccine, the first manufactured via plant cell culture. G-8 nations pledge billons for vaccine production.

The Pharmaceutical Industry has been slow in adopting radio frequency technology (RFID) to help control diversion and counterfeiting, according to a recent study by ABI Research (Oyster Bay, NY, www.abiresearch.com). In fact, only 10 drug products are expected to be shipped with RFID tags or smart chips embedded in the labeling in the coming year.

Merck wins approval for rotavirus vaccine as Sanofi ships investigational H5N1 vaccine to NIH and CDC explores new flue diagnostics and novel vaccine-production technology.

Dow AgroSciences Receives Regulatory Approval for Plant-Made Vaccine
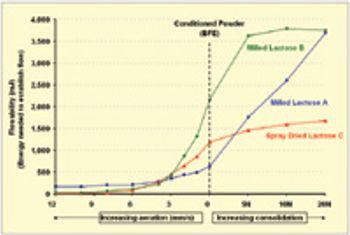
The pharmaceutical industry's focus on process understanding, monitoring, and control is driving manufacturers to take greater steps toward identifying possible manufacturing bottlenecks earlier in the development process. For tablet, capsule, and excipient producers, such efforts include taking a closer look at the flow-ability of their powders.