
CMOs may be gaining as strategic partners to large bio/pharma companies, but they have a much harder path to navigate.

CMOs may be gaining as strategic partners to large bio/pharma companies, but they have a much harder path to navigate.

Sharp acquired the pharmaceutical packaging facility in Bethlehem, PA.

GE Healthcare continues to ramp up its offerings in the bioprocessing space with the purchase of Asymptote and a continued partnership with Zenith Technologies.

FDA is in the center of the debate over developing and pricing new cancer therapies.
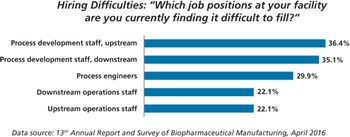
New study shows China biopharma companies face staffing shortages.

Advances in process analytical technology have been achieved, but significant challenges remain.

A case study reviews the reformulation and scale up of high drug load prototype using wet granulation process for a model formulation.

RSSL has added new equipment for measuring the surface area of powder particles, which is important for determining the performance of excipients and APIs.

The company has secured an additional facility in Hampton, Middlesex, as part of a project to expand its UK-based operations by 15% in 2017.

The agency is recommending the suspension of a variety of medications because of unreliable bioequivalence studies conducted by Micro Therapeutic Research Labs

Caladrius is selling the remaining percentage of the subsidiary in order to focus on cell therapy development.

A robust quality agreement and good communication scheme can help avoid and alleviate regulatory concerns.
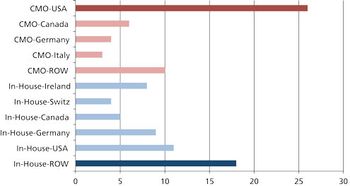
Moving global manufacturing operations may be more complicated than it appears.

Pharmaceutical Technology asked Siegfried Schmitt, principal consultant at PAREXEL, about the importance of quality agreements in the sponsor/contractor relationship.

The article reviews strategies for firms with or without existing in-house capacities and the pros and cons for outsourcing bio/pharmaceutical development and manufacturing.

This article reviews experiences with the outcome of in-house audits, audits by third parties, and purchased audit reports.

The industry is becoming more consolidated, but there needs to be some strategy behind the mergers and acquisitions.

SGS expands its elemental impurity testing services at its laboratory in Villeneuve-la-Garenne, France.

Quotient Clinical’s addition of CDMO QS Pharma increases the company’s footprint in the US an adds high potency molecule capability.

The outlook for the CMO and CDMO industry may be affected by ever-changing politics.

Analytical products for improved bio/pharmaceutical development.

Cold-chain requirements and the tight logistical windows needed for cell and gene therapies demand a focus on early communication and risk mitigation.
Andrea Zobel, senior director of product management, clinical trial supply, and logistics at PAREXEL International, examines key issues and questions that sponsors and contract partners must address.

Many pharmaceutical manufacturers were late in involving contract partners in serialization efforts. Are you ready, and are you working with the right partner?
As the date for implementation of the Drug Supply Chain Security Act approaches, bio/pharma companies and contractors should focus on key areas.