
Quality, innovation, and new approval pathways open drug development options for the Chinese market, including injectable contract manufacturing.

Quality, innovation, and new approval pathways open drug development options for the Chinese market, including injectable contract manufacturing.

Bio/pharmaceutical contract service provides continue to invest in development, facility upgrades, technological advancement, and mergers and acquisitions.

New approaches add flexibility and reduce risk for contract development and manufacturing organizations (CDMOs) and their clients.

Planning ahead is key to enabling a continuous and secure supply chain that adapts to changes in market demand.

Now past the “wait-and-see” standoff, most pharmaceutical companies and their contract partners are considering long-term requirements, including distributors’ needs.

The author reviews some of the key considerations when selecting a vendor and crucial parameters that must be defined in the tech-transfer process to ensure the greatest chance of success.

Training and mock audits are the key to preventing data integrity issues with partners offshore, but the process must start at home. Compliance consultant John Avellanet shares best practices and ways to minimize costs.

The company plans to purchase and develop an 18-acre site in Des Plaines, Illinois.

EAG Laboratories announces new company identity and intent to expand testing, analysis, and characterization capabilities across multiple markets.

Patheon launches initial public offering to repay outstanding notes and expenses.

Bio/pharma companies that are qualifying potential contract service providers should investigate post-merger integration activities and capital structure as part of the due diligence.
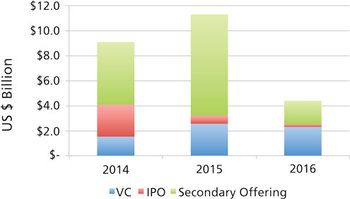
CDMOs need to be aware that unfavorable public markets put emerging bio/pharma R&D spending at risk in 2017.

CPhI Pharma Awards seek nominations for excellence in development and manufacturing.

Data integrity and cGMP issues demand closer scrutiny of suppliers. Bribery and corruption may become the next supply chain flashpoint.

The company expanded its services to include oligonucleotide API development and manufacturing and received approval for its Caponago manufacturing facility.

A RoTab tablet press can product up to 42,000 tablets per hour for Juniper Pharma Services.

Recipharm adds hardware, software, and cloud services for serialization compliance processes.

Brammer Bio establishes late-phase development and commercial manufacturing facility for advanced cell and gene therapies in Lexington, MA.

The new company is the product of a merger with Formex.

Uncertainty about the demand for a biologic medication can be partly mollified with some well-planned capacity outsourcing, contends a new report by ORC International sponsored by Patheon.

Industry experts discuss common considerations and recent technological advancements in blow-fill-seal technology.
Process analytical technology paved the way for continuous manufacturing.

Acquisition binges often lead to hangovers; here’s what to watch out for.

The company expanded its topicals capacity with an investment in the Becomix RW30 model homogenizer.

PharmTech sat down with an intellectual property lawyer to examine how companies are protected when they engage in activities where sharing of trade secrets must occur.