
WellSpring Pharma formed a strategic partnership with IDT Australia and invested $3 million in new equipment.

WellSpring Pharma formed a strategic partnership with IDT Australia and invested $3 million in new equipment.
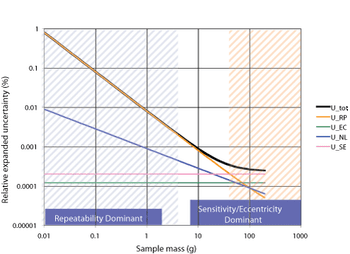
A program for calibration and routine testing of weighing instruments ensures accurate results.

Transferring the manufacturing of a drug from one scale to another or between manufacturing sites presents both technical and business challenges.
Manufacturing highly toxic compounds in a biopharmaceutical environment tests equipment and systems.

Demand is driving expansion and consolidation of formulation and clinical trial materials services.

At DCAT's annual meeting, Bill Downey, president of the market research firm High Tech Business Decisions, summarized results from its latest survey on biopharmaceutical outsourcing.

Collaboration will provide for unified development and manufacture of antibody drug conjugates.

Hovione will operate a commercial-scale continuous manufacturing facility in New Jersey as part of an agreement with Vertex Pharmaceuticals.

Global economic and political uncertainty could slow bio/pharma development activity.

The pharma outsourcing market starts 2016 with expansions, acquisitions, and new offerings.

As specialty API outsourcing grows, manufacturers and contract development and manufacturing organizations are investing for the long haul.

Data integrity has become a more serious compliance problem at pharmaceutical manufacturing plants throughout the world.

The author lists five key areas to consider when selecting a CDMO to develop highly potent formulations.
The authors provide their perspectives on shipping validation.

Cirrus Pharmaceuticals will launch services for manufacturing cGMP materials for early-phase clinical trials at its Raleigh-Durham, NC facility.

Recipharm acquires Mitim, adding scale and technology in injectable beta lactams.

PharmSource report addresses how the opportunity for antibody drug conjugates measures up to the bio/pharma industry’s expectations.

Smart glasses enhance remote troubleshooting and process management for pharmaceutical manufacturing.

3M Drug Delivery Systems, Ei Solutionworks, IDT Biologika join CMO/CDMO association

When implementing disposable technology for aseptic processing, considerations include material compatibility, material sourcing, facility layout, and training.

Heightened global uncertainty could slow bio/pharma development activity.Bio/pharmaceutical companies, and the companies that serve them, tend to think they are immune from broader macroeconomic and political developments. As populations age, emerging middle classes expand, and scientific knowledge progresses, research on new drugs and demand for new therapies seem to follow an inexorably upward trend.

Catalent plans a $4.6 million investment to expand secondary packaging and storage in Asia.

Sustainable harvesting combined with CMO expertise helped Centroflora CMS ensure supply continuity after it acquired Boehringer Ingelheim's non-captive API phytochemicals portfolio.

Steven Denham, director of biostatistics at MPI Research, discusses the impact the Standard for Exchange of Nonclinical Data may have on the pharmaceutical industry.

Mergers and acquisitions have changed the shape of the contract services market as big players seek to build full-service capabilities.