
SGS invested in test equipment for analyzing extractables and leachables at its New Jersey laboratory.

SGS invested in test equipment for analyzing extractables and leachables at its New Jersey laboratory.

Vetter expanded visual inspection facilities and controlled-temperature storage at its Ravensburg Vetter West facility in Germany.

WuXi AppTech acquired HD Biosciences, a preclinical drug-discovery-focused CRO.

Camfil Air Pollution Control has expanded its testing laboratory and added a dust collection test rig for the ANSI dust collection standard.

EAG Laboratories offers dermal absorption studies using OECD methods.

High Street Capital acquired Avomeen, a full-service chemical testing laboratory, in late December 2016

UK-based Orchard Therapeutics and PharmaCell ally to support clinical trials and commercialization of Orchard’s ex-vivo gene therapies.

Catalent announces a development agreement with JOT to evaluate softgel options for a resveratrol drug candidate.

Robust venture capital investment gives CDMOs and CROs a positive outlook for 2017.

Healthcare policies, R&D investments, and drug approvals will test bio/pharma.

Pharma Tech introduced a new high-speed bottling line to its facility in Union, MO.

AGC adds second biopharma contract manufacturer with acquisition of CMC Biologics.

Novasep’s three facilities located in France and Germany are free of Form 483.

The company has manufactured double-digit number of batches on the new filling line, for use in early-stage clinical trials.

BMS changes its US geographic footprint with R&D investments and closures.

At the American Society of Hematology Annual Meeting, Novartis and Kite Pharma both released results from early-phase clinical trials with CAR-T therapies.

M&A activity, new business models, fundraising limits, offerings from small CDMOs, and combination products are driving decisions in the contract services market..
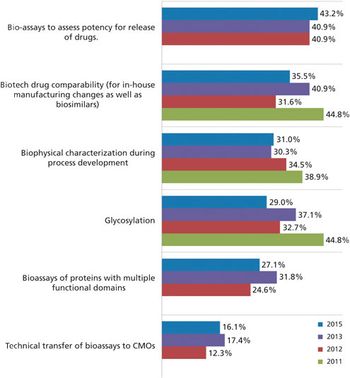
Biosimilars may be the key to CMO growth.

Recipharm and Laccure AB signed a commercial collaboration for the manufacture and delivery of Laccure’s bacterial vaginosis treatment.

Vetter’s Ravensburg data processing center received certification from a German industrial testing organization, certifying that the company’s customer and process data are protected.
Understanding of endotoxin assays and a range of detection technologies are essential for effective testing.

Idifarma has acquired a Bosch GKF-702 capsule filling machine that can manufacture 3000 to 42,000 capsules per hour.

Agilent’s new facility in Folsom, California includes laboratory, order fulfilment, and warehousing space.

Catalent adds two softgel facilities and packaging capabilities with acquisition of Canada-based Accucaps.

Patheon adds API manufacturing capacity with acquisition of Roche’s Florence, SC facility.