
Careful mixing during a product's distillation can help avert trouble from a strong concoction.

Careful mixing during a product's distillation can help avert trouble from a strong concoction.

Researchers develop various catalytic approaches for improving yield, purity, stereoselectivity, and process conditions.
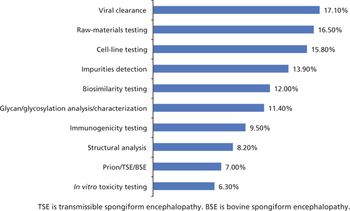
Biosimilar manufacturers need better expression systems and analytical tools to compete.

Russia is aiming to provide an alternative to China and India for drug manufacturing, including APIs.

The authors propose a plan to keep the US pharma industry afloat and in the lead.

Recent recalls have contributed to the pharmaceutical industry?s heightened awareness of glass delamination (i.e., the formation of glass flakes in a vial), which could affect drug quality and patient safety. To confront this growing problem effectively, drugmakers must understand its causes.

John Kelly, vice-president of strategy and transitioning sites for Pfizer Global Supply, discusses the company's manufacturing and supply strategy and network.

Getting the most value out of M&As requires proper upfront legwork.

Expanding the organization's mandate will strengthen inspections.

The authors examine risk management relating to the quality issues of clinical-trial materials and discuss areas that would benefit from additional consideration and harmonization.
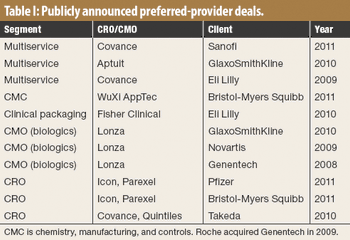
CROs that have made big acquisitions could be outmaneuvered by evolving sourcing models.

A Q&A with Rao Tatapudy, vice-president of scientific affairs at Catalent, on recent industry trends.

Letting contamination build up can cause multiple headaches.

In an effort to balance bilateral trade, India is urging China to increase Indian pharmaceutical imports.

Manufacturers fund research and reduce prices to tackle diseases.

The global excipients market shows moderate growth, increased consolidation, and expansion activity in emerging markets and select product areas.

With the Western world recovering slowly from a recession, and tighter restrictions for medical products being applied by the FDA and other regulatory authorities, more medical device R&D is being located in Asia.

The contract services industry is a crowded market and it's important for companies to differentiate themselves to stand out.

The past year has seen significant consolidation of mid-size CROs, with many of them being bought or put up for sale. In particular, the top six or seven CROs seem to be signing more preferred partner deals, such as Pfizer teaming with Parexel and ICON, and Takeda?s deals with Covance and Quintiles, which is driving mid-size CROs growth through major acquisitions.

Although the European market is approximately 50% smaller than the US in terms of landmass, the population of Europe is approximately 50% larger and this presents a huge market opportunity.

Oriol Prat, Marketing Manager, and Marga Viñes Senior Product Manager, from Grifols give us a quick rundown of how business is changing and what challenges the company faces.

In a quick-fire interview, Dr Stephen Taylor, Vice President & Commercial Director at Fujifilm Diosynth Biotechnologies, looks at some of the challenges facing biologics outsourcing.

A Q&A with John Plachetka, chair, president, and CEO of POZEN, on recent industry trends.
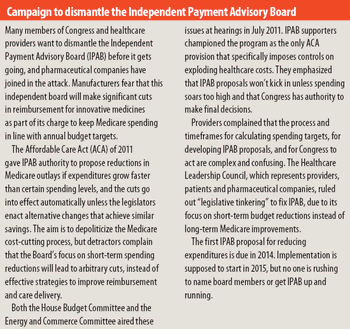
PDUFA renewal legislation sets stage for new policies affecting revenue, resarch, and oversight.

Being aware of a forthcoming inspection or how a product was made can make a huge difference.