
For a drug-development process that relies on outsourced services, special considerations are needed to ensure the proper transfer of technology and information from one phase to the next.

For a drug-development process that relies on outsourced services, special considerations are needed to ensure the proper transfer of technology and information from one phase to the next.

PharmaCell enters an agreement purchase TiGenix therapy production facility.

Regis Technologies passes a recent FDA audit with no Form 483 observations.

With numerous biologics set to come off patent soon and the percentage of new therapeutics based on biomolecules growing, demand for contract manufacturing in the biopharma space is heating up.

The R&D model is in transition and creating new demands on contract services providers.

Roger Hayes of MPI Research discusses highly potent drug development.

Siegfried Schmitt, principal consultant at PAREXEL, discusses the state of drug manufacturing in India.

Ongoing changes create new opportunities for CROs and CMOs.

Brian Galliher of Cook Pharmica discusses visual inspection systems.

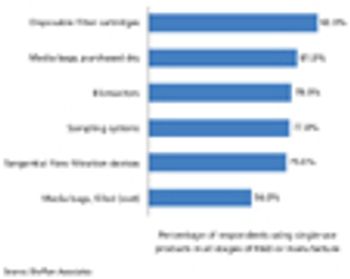
Data from BioPlan Associates’ 10th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production suggest that the interest in disposable devices has begun to extend to biopharma operations beyond basic single-use bags and connectors.

Recipharm has invested EUR 32 million ($43 million) in its Wasserburg, Germany site to expand lyophilization capacity.

Practicality of implementation should be a part of vision in the bio/pharmaceutical industry.

Through its educational and networking opportunities, the American Association of Pharmaceutical Scientists plays an important role in partnering throughout the drug- development and commercialization process.

New partnership aims to eliminate months from the typical transition time required to move chemistry from the laboratory into commercial applications.
Advances in techniques and single-use systems are revolutionizing vaccine manufacturing.

A recent survey shows that talent retention is an issue on industry level for contract research organizations (CROs), particularly for CROs located outside the United States.

Are strategic partnerships in clinical research a model for CMC services?
Industry experts share their views on the outsourcing model and the current and future direction of contract chemical API manufacturing.

A CMO perspective on the CMO-client partnership.

Advances in techniques and single-use systems are revolutionizing vaccine manufacturing.

Fast Locator: 2013-2014 Europe and Asia

2013 contract research and manufacturing services in North America.

View companies' outsourcing profiles from PharmTech's 2013 Outsourcing Resources Fast Locator Index in the following categories.

Recent draft guidance from FDA on contract manufacturing and quality agreements highlights the importance of such agreements and define the roles and responsibilities of each party to be in keeping with quality risk-management principles.

Rx-360, a pharmaceutical industry supply-chain consortium, is advancing approaches between pharmaceutical companies, suppliers, and contract manufacturers to better secure the pharmaceutical supply chain.