Increased pharmaceutical trade creates new challenges for regulatory oversight.


Increased pharmaceutical trade creates new challenges for regulatory oversight.

Claudia Roth, President of Vetter Development Service USA, discusses trends in single-use technology for clinical manufacturing.

Understanding the supply-chain challenge and coupling high-efficiency chromatographic techniques with information-rich detectors are leading to improvements in the management of extractables and leachables in parenteral drugs.

Contract service providers expand capabilities in API and finished product manufacturing to meet demand for high-potency drugs.

The industry may not be ready for India and China as regulatory issues emerge.

The US and EU move forward with measures to fortify the pharmaceutical supply chain.

Since its inception in 2011, Fujifilm Diosynth Biotechnologies has added capabilities and single-use production capacity for mammalian-derived product manufacturing, upgraded development and pilot-scale microbial laboratories, and installed advanced analytical instrumentation.

Roberto Darienzo, chief operating officer of Halo Pharmaceutical, describes strategic plans and industry trends.
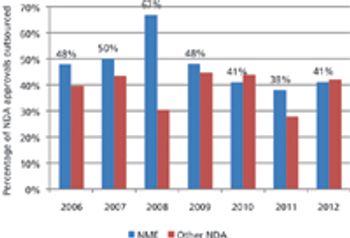
Approvals of new drugs are on an upward swing, but only a few CMOs are benefiting.

A Q&A with Stuart E. Needleman, President and Chief Operating Officer of Aptuit, on recent industry trends.

Outsourcing is weighing in more as a tactic for cost-cutting, but it is still not the primary weapon.

Eisai and Biogen Idec pursue an innovative approach to capacity management.
As the strategic value of emerging markets increase, pharmaceutical companies increase their R&D and manufacturing investments.

FDA's requirements for API manufacturers in regards to ICH Q7.

Growth is seen in outsourcing of insect- and plant-cell-based bioproduction expression systems.

Is the contract-only CMO an endangered species?
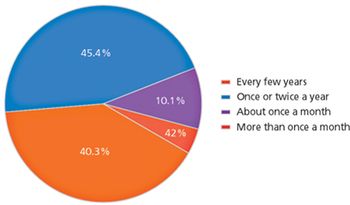
A UC Berkeley survey provides insight into biopharma's risk concerns and strategies.

CROs are keeping pace with the increased globalization of the biopharmaceutical/pharmaceutical industry through a combination of acquisitions, partnerships, and select investments.

In the arena of pharmaceutical outsourcing, when speaking of partnerships, the discussion typically focuses on the relationship between a pharmaceutical/biopharmaceutical company as the sponsor company and a contract-service provider.

Preferred-provider relationships are transforming the bio/pharmaceutical industry.

When speaking of partnerships, the discussion typically focuses on the relationship between a pharmaceutical/biopharmaceutical company as the sponsor company and a contract-service provider.

Adherence to GMP in API manufacturing is crucial in determining the safety of drug products.

Innovation resulting in improved productivity continues unabated and is a primary driver for many of the current biopharmaceutical trends.

Will international biomanufacturing outsourcing become mainstream in this decade?
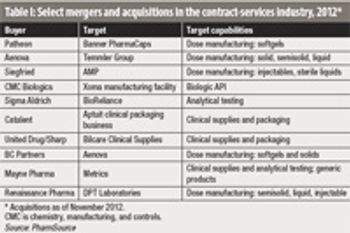
The quest to build critical mass and broaden capabilities has been a key factor in deal-making.