
Consider these tools and strategies for optimizing the manufacturing process.

Consider these tools and strategies for optimizing the manufacturing process.
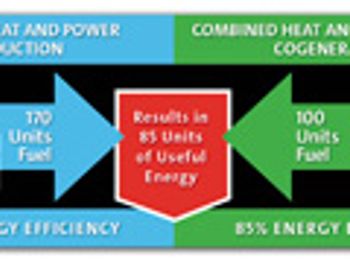
Today's pharmaceutical companies are striving to reduce costs and maximize efficiencies, and must make decisions on the best way to deploy their limited resources.

Working together affords many unseen opportunities for pharmaceutical innovation.

Measuring the size of the market for contract manufacturing services requires a careful hand.

The weak global economy adds to the challenges of bio/pharma companies and their suppliers.
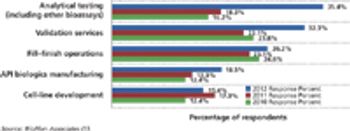
Budgets for biopharmaceutical activities are gaining in select functional areas except outsourcing.

The author provides results and commentary on a survey analyzing outsourcing strategies, practices, challenges, and outcomes in the selection of a CRO.
An examination of the current and projected market for biosimilars, development costs for biosimilars compared with small-molecule generic drug, and partnerships in biosimilars.

The author discusses how a CDMO helps in gaining process understanding and in developing robust, high-quality products and processes.

The authors highlight costs, benefits, and implementation success factors across first, second, and third generations of facility management outsourcing contracts.

AMRI, a contract research and manufacturing organization, discusses its adaption of an insourcing model with Eli Lilly.

Annual survey shows strong growth for service providers and promises to continue into 2013.

Jim Miller, president of PharmSource, examines the future direction of CROs/CMOs and the factors influencing the pharmaceutical contract services sectors.

The past three decades have driven a purchasing evolution to a procurement revolution.

A Q&A with Thomas E. D'Ambra, Chairman, CEO, & President of AMRI, on recent industry trends.

Taking time to appreciate the industry's greatest achievements will inspire growth ahead.

Companies roll out expansions in manufacturing high-potency APIs and finished products.

Sponsor companies' increasing focus on strategic outsourcing has changed the rules of the game.

Presidents of leading associations offer views on the industry's future.
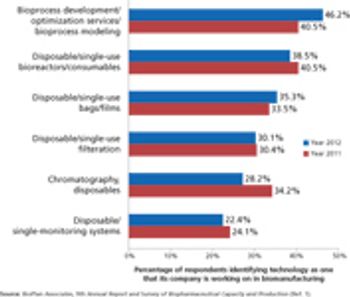
Industry wants more innovation, but can suppliers meet customer needs?

Highlights included the latest in pharmaceutical packaging equipment, containers, and labels and new capabilities among contract service providers.

Clinical research organizations see reform in clinical-trial process, including the establishment of chief innovation officer at FDA.

Recovery audits and other past practices in procurement can improve the bottom line.

Apple's experience with manufacturing facilities in China present opportunity for future best practice.

Service providers must focus on delivering a superior customer experience.