
A holistic approach to validation and quality assurance is essential.

A holistic approach to validation and quality assurance is essential.

An optimal engineering design is crucial for aseptic operation and cleaning.

Excipients must be carefully chosen to ensure optimum protection for vaccines and live biotherapeutic products.

The new line has the ability to aseptically fill powder, liquid, suspension, and combination forms into vials in clinical or commercial batch sizes.

Dimethyl sulfoxide is increasingly used in high-risk parenteral and medical device applications that must be manufactured as sterile products in their finished form. A study evaluated the effects four sterilization techniques have on the product quality of this ingredient.

CARBOGEN AMCIS has announced significant investments to increase manufacturing capacity in Switzerland and France.

The new facility in Grand Rapids, MI, is part of the CDMO’s aggressive expansion plan.

SiO2 Materials Science has received $143 million from the US government to accelerate capacity scale-up of its advanced primary packaging platform.
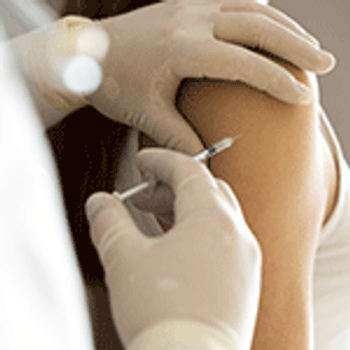
The authors compare formulations containing citrate with other buffers in reducing subcutaneous injection-site pain and discuss a formulation and excipients selection strategy.

Regulators have exaggerated expectations for simulated media fills.

Civica Rx plans redundant manufacturing capacity to relieve and prevent shortages of generic, sterile injectable drugs.

Consider equipment design, transfer systems, and maintenance when operating isolators for sterile manufacturing of pharmaceutical products.

The company showcased its compact robotic nest filling machine at CPhI Worldwide 2019 on Nov.–7 in Frankfurt, Germany.

Trends affecting primary pharmaceutical packaging include the shift to more complex drug-device combination products.

Through a partnership with the Sobti family, the CDMO will expand is injectable dosage form capabilities.

A recent report released by an FDA task force highlights the financial, manufacturing, and policy issues underlying the drug shortages of important prescription medicines in the United States.

The approval marks the first time a new glass composition has been approved by FDA since the approval of borosilicate glass more than 100 years ago.

AstraZeneca received approval from FDA for Fasenra Pen, a pre-fillled auto-injector pen that allows for self-administration of its asthma biologic therapy, Fasenra (benralizumab).

The company will supply its heparin sodium injection in prefilled syringe form.

Equipment and systems for aseptic transfer and manufacture meet cGMP and Annex 1 requirements for pharmaceutical fill and finish.

The companies will work together to produce enFuse, a patient-administered subcutaneous delivery device.

Glass vials have come a long way from mere commodities and are now considered an integral part of final drug product.
Formulators of parenteral drugs must be cautious of specific considerations and challenges that arise during development and manufacture.

Using Lean, Six Sigma, and other Op Ex practices is helping one contract development and manufacturing organization (CDMO) increase efficiency and optimization.
Leachable silicone oil may have an effect on large-molecule APIs, making it important to establish a robust analytical method to detect and quantify the substance.