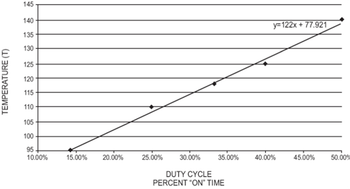
Preheating pinch valves prevents drift in the volume of liquid dispensed.

Preheating pinch valves prevents drift in the volume of liquid dispensed.

Consider the 3 Ps (product, process, and patient) when choosing a parenteral packaging material.

The syriQ Rigid Cap (SRC) and SCHOTT TopPac Rigid Cap (TRC) closure systems from Schott use an intuitive twist-off mechanism for ease of use and container closure integrity.
Expanded systems integration, serialization, and supply chain connectivity will enhance productivity and support counterfeit prevention.

Parenteral packaging of the future will include more automated lines, ready-to-fill packaging formats, and supply-chain transparency.

Microorganism lethality requirements for process validation must always be balanced with the need to protect product integrity and patient safety.

Baxter expands capacity for lyophilized cytotoxic oncology therapies at its fill/finish facility in Halle, Germany.
Delamination of glass packaging is a source of particulates in parenteral drugs, but identifying the root cause allowed the design of an improved manufacturing process for glass vials.

Although shortages, quality, and regulatory challenges remain, improved technologies and new investments suggest that the worst may be over.

Miriam Beyer, European marketing manager, West Pharmaceutical Services, describes causes of recent parenteral drug shortages.

Scottish injectable-drug manufacturer Symbiosis Pharmaceutical Services plans to expand its sterile filling facility.

The challenge of achieving zero visible defects (i.e., particulates) in parenteral drugs will require a coordinated effort at all stages of the supply chain, particularly in the production and filling of primary containers.

Manufacturers of parenteral drugs face challenges to increase efficiency, control particulates, control extractables and leachables, and eliminate product/package interactions; new containers and packaging equipment offer increased options.

GEA’s Pony NS2006L is a self-contained, high-pressure laboratory and pilot plant homogenizer for product development of advanced fluid applications.

FDA warns that compounded or repackaged drugs stored in certain syringes made by Becton-Dickinson may lose potency because of an interaction with the rubber stopper.

Operator attention to detail and adherence to procedures are crucial for proper cleaning.

GEA's self-contained homogenizer is designed for laboratory applications, including cell dispersions.

The company’s Scotland-based facility has successfully completed a scheduled inspection by the UK’s MHRA; there were no critical or major observations noted.

Flexion will have a dedicated manufacturing suite at Patheon's Swindon, England facility for Flexion's osteoarthritis steroid drug product.

Teva Parenteral Medicines initiates voluntary nationwide recall of select lots of Adrucil due to particulate matter.

Richard Johnson, President and CEO of the Parenteral Drug Association (PDA) speaks with Pharmaceutical Technology.

Presenters at IVT's Microbiology Week discussed best practices and recent guidance publications for microbial control in sterile and non-sterile pharmaceutical processes.

OXY-CAPT brings together properties of plastic and glass containers through the combination of oxygen absorption and cyclic olefin copolymer (COP) high barrier layers.

Symbiosis Pharmaceutical Services is now offering a new bulk lyophilization (freeze drying) service in response to increasing demand in the manufacture of bulk intermediates and APIs by lyophilization.