
Although technical paths for continuous solid-dosage manufacturing have been laid out and equipment and control systems have been developed, industry is slow to move forward.

Although technical paths for continuous solid-dosage manufacturing have been laid out and equipment and control systems have been developed, industry is slow to move forward.
Design of experiment plays a crucial role in the optimization process of formulation development.

Anil Kane, executive director, Global Head of Formulation Sciences, Pharmaceutical Development Services at Patheon discusses key parameters in the development and manufacturing of oral solid-dosage forms.
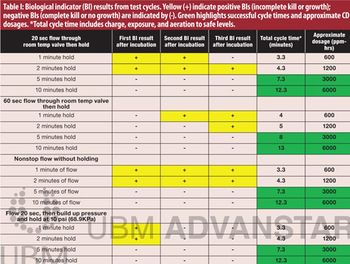
A chlorine dioxide sterilization cycle was developed for a novel split-valve aseptic powder transfer device.

A venture between GEA and Siemens aims to familiarize more pharmaceutical companies with more modern control and continuous processing.

This novel technology was developed in response to challenges involved in conventional manufacturing of multilayer tablets, including in-line control of the tablet weight, the tendency to delamination, direct contact between the two tablet layers, and cross contamination.

This article summarizes the evolution of the viscosity standards and their corresponding applications in the USP−NF compendia.

A case study reviews the reformulation and scale up of high drug load prototype using wet granulation process for a model formulation.

Manufacturing will be carried out at the Pfizer Newbridge, Ireland, facility, which is now part of Pfizer CentreOne’s contract manufacturing network.

Improved process analytical technology and new ways of thinking seek to enhance measurement and control for next-generation pharmaceutical manufacturing.

Soft sensors are powerful tools that can be used along with spectroscopic instruments in on-line measurement.
This article introduces the concepts of pooled variance and the central limit theorem, which are intended for establishing acceptance criteria for blend uniformity data of granular powder blends when a significant degree of sampling bias is involved.

Camfil Air Pollution Control has expanded its testing laboratory and added a dust collection test rig for the ANSI dust collection standard.

GEA’s ConsiGma continuous tableting line combined with Siemens’ automation and Sipat data management systems enables continuous manufacturing.

Catalent announces a development agreement with JOT to evaluate softgel options for a resveratrol drug candidate.

Moisture uptake during the end-to-end manufacturing process and supply chain can affect product quality. Simulation tools based on mechanistic models help define storage and handling requirements for oral solid-dosage drugs.

Advances in materials and equipment for pharmaceutical blister packaging protect quality and enhance shelf life.

Principles of dissolution testing, including method development and testing apparatus, are reviewed.

FDA and BARDA awarded a contract to Continuus Pharmaceuticals to develop an end-to-end continuous manufacturing process for solid-dosage drugs.
Hot-melt coating was used to develop taste-masked orally disintegrating granules of acetaminophen and caffeine.

Catalent adds two softgel facilities and packaging capabilities with acquisition of Canada-based Accucaps.

A lifecycle approach can be used to develop GMP-compliant cleaning procedures for continuous manufacturing of solid-dosage pharmaceuticals.

Shear-sensitive ingredients in a tableting formulation experience different conditions in a batch tumble blender than they would in a continuous paddle blender.

A dissolution method should have adequate discriminatory power to detect formulation changes that affect the dissolution rate of a drug product.
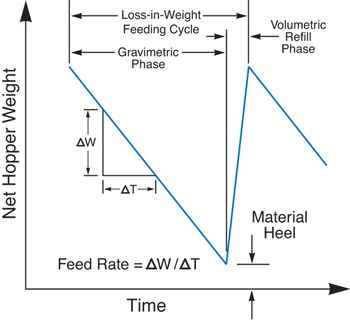
Designing loss-in-weight feeders for accurate and consistent refill is crucial to a continuous solid-dosage process.