
PharmTech’s 2016 survey shows general satisfaction with existing solid-dose and parenteral manufacturing equipment, and slow adoption of continuous manufacturing processes.

PharmTech’s 2016 survey shows general satisfaction with existing solid-dose and parenteral manufacturing equipment, and slow adoption of continuous manufacturing processes.


The agency provides quality, development, manufacturing, and labeling recommendations.

This article presents recommendations based on a program that was set up to qualify members of a large, diverse team at one oral solid-dosage-form manufacturing facility.

A US government report on advanced manufacturing promotes continuous manufacturing of pharmaceuticals, which has had recent commercial success but faces challenges for widespread adoption.
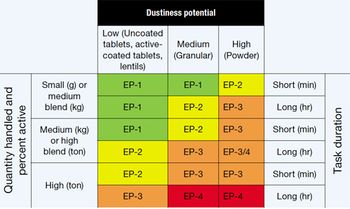
Safe handling of HPAPIs requires determining exposure potential and selecting appro-priate containment strategies.

Armin Gerhardt, associate professor of Pharmaceutical Science, Concordia University Wisconsin School of Pharmacy, discusses the effects of moisture on product quality and how to achieve good control of moisture during pharmaceutical manufacturing operations.

FDA approved an update in the manufacturing of Prezista (darunavir) using a continuous manufacturing line at Janssen Supply Chain’s facility in Puerto Rico.

Texture analyzers can be used to evaluate wall hardness and elasticity of softgel capsules.

Industry experts and FDA’s Office of Pharmaceutical Quality discuss the challenges, trends, and regulations involved in ensuring quality in solid and semi-solid dosage forms.

Pharmaceutical Technology spoke with Bill Randolph, vice-president, Technical Services, Janssen Supply Chain, about some of the considerations for technology transfer of a continuous, solid-dosage manufacturing process and what he sees as the outlook for continuous manufacturing.

Pharmaceutical Technology spoke with FDA to get the agency’s insights on how the industry can ensure quality in solid and semi-solid dosage products.

The author discusses key areas of focus and presents best practices from the International Pharmaceutical Excipients Council (IPEC).

Wear-resistant materials and coatings protect tablet punches and dies from abrasive formulations.

Hovione will operate a commercial-scale continuous manufacturing facility in New Jersey as part of an agreement with Vertex Pharmaceuticals.
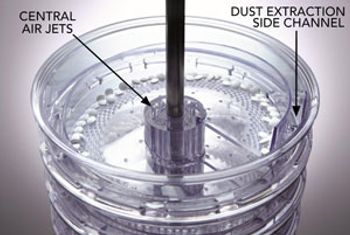
Dedusting equipment and techniques address problems associated with tablet manufacturing dust accumulation.

Tablet presses require regular inspection and maintenance to prevent premature wear and tableting problems.
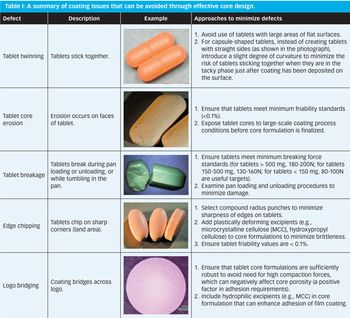
Common defects in tablet film coating can be minimized by effective design of the tablet core and the coating process.

Hermes Pharma has commercially implemented hot-melt coating (HMC) technology at its production facility.

With the acquisition of Medipac’s tube filling assets for effervescent tablets, Romaco can now offer the complete line configurations for effervescent tablets.

Integrated pharmaceutical blister-packaging equipment systems strengthen serialization and brand protection capabilities.
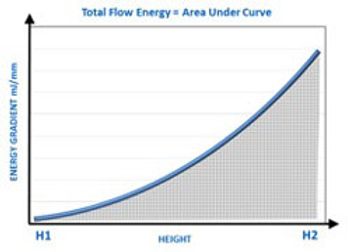
Dynamic powder testing and measurement of bulk powder properties can complement shear cell testing to identify the causes of poor hopper performance in solid-dosage drug manufacturing.

Experts at the ISPE annual meeting describe best practices, including containment and production in classified spaces.

AstraZeneca’s solid-dose facility in Taizhou, China received ISPE’s FOYA 2015 Overall Winner award.

A rheometer and powder flow tester can measure how pharmaceutical materials, such as powders from a hopper, flow.