
CPI, the University of Strathclyde, GSK, and AstraZeneca Collaborate on a continuous direct compression digital twin for pharmaceutical formulation optimization.

CPI, the University of Strathclyde, GSK, and AstraZeneca Collaborate on a continuous direct compression digital twin for pharmaceutical formulation optimization.

Establishing OEL data and ensuring appropriate engineering controls are crucial aspects of safe handling.

High-throughput platforms can be used to develop tertiary phase diagrams, which can be leveraged to identify the most stable SEDDS formulations and excipients for lipid-based drug delivery systems.

PSE, a supplier of advanced process modeling software and services, plans to become part of Siemens Digital Industries.

At-line NIR measurements can replace a laboratory HPLC measurement for API content in oral solid-dosage drug manufacturing.

Early adopters of continuous manufacturing approaches shared their plans and some of their experiences at the 11th annual Charles Jarowski Symposium in Industrial Pharmacy.

Marchesini Group’s Compact 12 electronic counter with HarleNIR vision system measures product and active ingredient when filling and capping bottles for tablets and capsules.

Lonza plans to implement MES software to accelerate paperless, 24/7 production of drug capsules.

Suheung’s new facility in Vietnam is dedicated to vegetarian capsule manufacturing.

An agreement with Sanofi gives Catalent access to commercial spray drying facility in Haverhill, UK.

StarTab from Colorcon was designed to ensure functionality and stability for direct compression tableting.

Expansion at the Eberbach, Germany facility includes two Vegicap encapsulation lines, printing, inspection, and packaging capabilities.

Market demand and regulatory guidance continues to promote improved medication design.

Understanding the API, delivery mechanism, and excipient functionality is essential to solving drug solubility challenges.

Containment valves and smart monitoring can keep employees safe and improve manufacturing efficiency when handling potent APIs, intermediates, and solid-dosage drugs.

Optical coherence tomography can improve quality control and development of coated dosage forms by allowing film thickness to be measured in real time.

This paper describes how the concept of acceptance value can be redefined to remove bias and more closely reflect quality targets.

Innovative technologies, such as drug-loaded devices and 3D printing, enable advances in implantable devices and other novel dosage forms.
Lipid-based formulations offer a means of addressing the physicochemical and biological challenges of poorly soluble APIs.

3D printing is being explored as a manufacturing method for on-demand, personalized medicine.

Senomyx uses an approach, known as taste-blocking, to address the challenges of bitter APIs. Kenneth J. Simone, vice-president of Pharmaceutical Business Development, Senomyx, speaks with Pharmaceutical Technology about this technology.
The level of tastemasking required will depend on the API properties and the dosage form design.
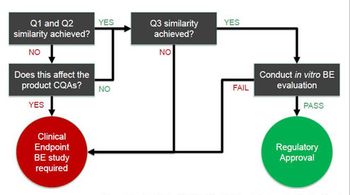
This article examines IVBE testing requirements for topical creams and explores some of the analytical techniques necessary.

API-in-capsule approaches enable pharmaceutical companies to quickly assess new drug candidates with reduced API consumption and to increase speed to clinic.

This article describes the approaches used during the development of a dexlansoprazole delayed-release orally disintegrating tablet (ODT) to evaluate tablet size and texture as they relate to disintegration rate and patient experience; in addition, the resistance to alcohol was also characterized.