
A technical forum featuring Tim Freeman of Freeman Technology and Carl Levoguer of Malvern Instruments.

A technical forum featuring Tim Freeman of Freeman Technology and Carl Levoguer of Malvern Instruments.

It's better to catch costly mistakes in the laboratory before they reach the accounting department.

Industry experts working with extended-release injectables discuss challenges and solutions to formulating and manufacturing these complex products.

The author outlines the scientific aspects of forced degradation studies that should be considered in relation to ANDA submissions.
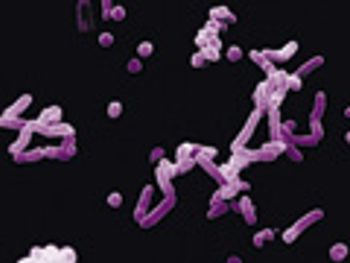
In this technical forum, experts describe different methods of rapid microbial testing and their applications.

An analytical technique that is receiving increased attention in the pharmaceutical industry is atomic force microscopy. We interview Mark Leaper from the UK's De Montfort University to find out more about this technology.

Parametric release and real-time testing use manufacturing data to ensure that products are made according to defined standards. PharmTech talks to Boehringer Ingelheim's Heribert Hausler about these issues.

The authors provide a review of test methodology and standards, including current industry and regulatory proposals, for biological indicator growout times.
Readers share their views on bioprocessing challenges, equipment use, and outsourcing trends in our annual bioprocessing equipment and processing survey.
In Part III of a three-part article, the authors examine various degradation routes of APIs, impurities arising from interactions in formulations, metabolite impurities, various analytical methods to measure impuritie, and ways to control impurities.

New product reviews for April 2012.

How to use geographic diversification and legacy technology transfers to avoid product shortages.

Failure to disclose info may work sometimes, but eventually every question will be answered.

Understanding the differences between convenience, target, and self-selected samples.

In a world where product recalls can mean the end of a company, all batches must be perfect.

Genotoxic impurities and how to identify them and control for them have been a concern for several years in the pharmaceutical manufacturing industry. Pharmaceutical Technology spoke with Bo Shen, PhD, principal scientist at Amgen and chair of the AAPS Pharmaceutical Trace Impurities Focus Group, to gain insight on key challenges.

In Part II of a three-part article, the authors examine impurities from chiral molecules, polymorphic contaminants, and genotoxic impurities.

New product reviews for March 2012.

A nickel's worth of free advice to the competition could come at the expense of your bottom line.

In Part I of a three-part article, the authors discuss what constitutes an impurity and the potential sources of impurities in APIs and finished drug products.
Where is the variability coming from and what have we done to minimize it?

The Asian nation is strategizing to take the lead over its regional competitors in pharmaceutical exports.

New product reviews for February 2012.

In Part I of a three-part article, the authors discuss what constitutes an impurity and the potential sources of impurities in APIs and finished drug products.

Technology may expedite operations, but the absence of the human element could cost dearly.