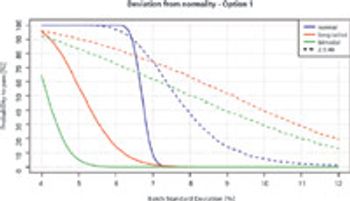
New European Pharmacopoeia chapter aims to resolve problems with applying the harmonized UDU test to large sample sizes.

New European Pharmacopoeia chapter aims to resolve problems with applying the harmonized UDU test to large sample sizes.
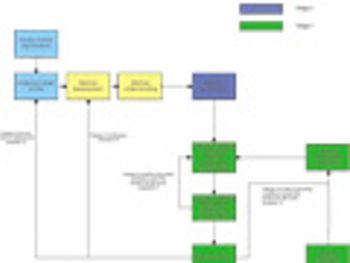
The authors describe how traditional approaches to analytical method and validation may benefit from alignment with quality-by-design concepts.

Potency is a required measurement to determine the amount of active ingredient contained in a preclinical dose formulation. Assessing potency ensures that the test system receives the appropriate amount of active ingredient based on predetermined specifications.

Real-time experimentation may offer continuous process improvement.

New product reviews for October 2012, featuring analytical equipment.

International trade can be great for business, but breaking border laws can put one in hot water.

Industry experts share perspectives on analytical instrumentation, methods, and data analysis.

MIT survey results address product and site characteristics that statistically correlate with quality performance.
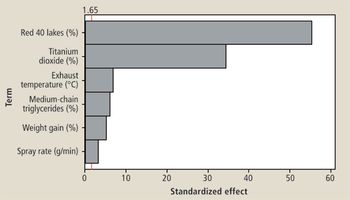
The authors describe a QbD study that was performed to optimize a coating system.

New product reviews for August 2012.

The author discusses how to manage pending pharmacopeial changes.

Applying the recommendations of ICH Q10 to statistical analysis can help prevent product recalls.

Meticulous system configuration can prevent machines from taking over.

The authors, part of the International Consortium on Innovation and Quality in Pharmaceutical Development (IQ Consortium), explore and define common industry approaches and practices when applying GMPs in early development.

Cleaning validation provides assurance that the quantity of residual substances collected from equipment surfaces are within permissible limits, helping to ensure quality control and safety in pharmaceutical manufacturing facilities. Three different cleaning validation methods for measuring the carbon in residual samples of various pharmaceutical substances were compared.

Experts share insights into analytical tools and techniques.

We have developed an in-house broth to neutralize a preservative for traditional microbiological testing following USP <61> Microbiological Examination of Non-Sterile Products: Microbial Enumeration Tests. We would like to use a rapid method to release product faster, but is there a broth that will be effective on our products?

Industry experts share their insight on solid-dosage and sterile manufacturing.

IQ Consortium representatives explore industry approaches for applying GMPs in early development.

Careful attention to detail will help to prevent valuable assets from "melting" away.

An industry roundtable on how users and makers can best assess and manage black specks.

Applying current principals to traditional factorial designs.

Uniform dose formulation is key to meeting safety study requirements.

Industry experts share perspectives on analytical instrumentation, methods, and data analysis.

An understanding of the pan-coating process based on first principles can support successful scale up.