
Equipment purchased today for the packaging line should be serialization-ready in preparation for upcoming requirements.

Equipment purchased today for the packaging line should be serialization-ready in preparation for upcoming requirements.
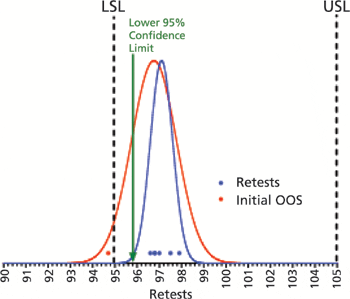
Two methods to evaluate retest data following out-of-specification results.

Even in an industry in which all product development is complicated by the intricacies of human biology, orally inhaled products (OIP) stand out as singularly demanding.

Recent activity in standards-setting organizations has raised interest in the impact of testing for impurities that may enter the product before it is mined or harvested or even due to intentional use of some reagents.

Revisions to the United States Pharmacopeia (USP) General ChapterHeavy Metals have been much discussed over the past decade.

Advances in data loggers and radio-frequency identification tags help meet the increasing need for managing the pharmaceutical cold chain.

The authors discuss the valuable information that can be obtained from indexing and its applications in routine screening and analysis of solid forms.

Tim Freeman of Freeman Technology explains how new analytical technologies have influenced quality criteria and standards for the uniformity of dosage units, and why more accurate systems are leading to greater focus on tablet scoring.

Magnetic separation equipment reduces ferrous metal contamination.

A few years ago, drug criminals would put all their efforts into matching packaging and labeling, or manufacturing good-looking fake materials. Today, criminals are capable of much more.

Can postapproval FDA filings immunize pharma companies from patent lawsuits?
A change in terminology could emphasize patient protection.

As-found data is a crucial component of pipette calibration for laboratories that must comply with GMP regulations.

FDA announced the availability of the guidance “Q11 Development and Manufacture of Drug Substances†in the Nov. 20, 2011 edition of the Federal Register.

Experts share insight into extractables and leachables testing, including high-risk products, analytical testing, and regulatory requirements from FDA and EMA.

A two-day workshop on the "science behind pharmaceutical stability" was held in conjunction with the Annual Meeting of American Association of Pharmaceutical Scientists (AAPS) on Oct. 21-22, 2011 in Washington, DC.
To support global stability practices, fundamental basics must be in place.

This article introduces"mean kinetic relative humidity" for evaluating the impact of humidity variability.
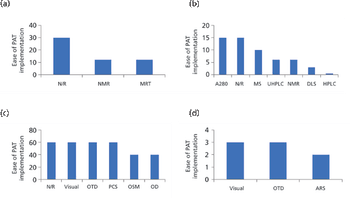
In this paper, the authors review the various analytical methods that can enable use of PAT.

New systems that combine Raman spectroscopy with automated imaging support the efficient gathering of such data, including information concerning size and shape distributions for individual components within a formulation.

Hydrogen deuterium exchange by mass spectrometry is a powerful analytical approach that can be used to map higher order structures of proteins.

This discussion aims to outline an approach to metal contamination prevention that should achieve a level of control acceptable to all stakeholders.
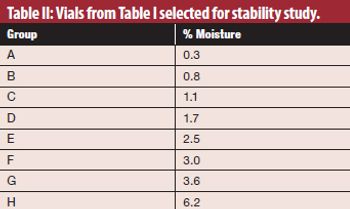
Using an alternate moisture-generation method may provide more accurate data for regulatory submissions.
The authors describe a process for generating high affinity, fully human antibodies in culture.
This article focuses on the growing need for effective data management in the life sciences industry-especially among smaller pharmaceutical manufacturers.