
Chemical imaging of solid dosage forms has become a powerful analytical tool for the development of solid dosage forms.

Chemical imaging of solid dosage forms has become a powerful analytical tool for the development of solid dosage forms.
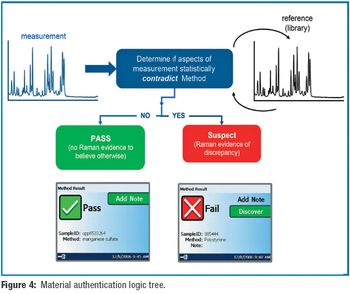
Using a handheld Raman spectrometer, the authors developed methods for 28 commonly used excipients and active ingredients.

The selection of an appropriate salt form for a potential drug candidate is an opportunity to modulate its characteristics to improve bioavailability, stability, manufacturability, and patient compliance.

Editors' Picks of Pharmaceutical Science & Technology Innovations; Analytical system provides multiuser capability; Encapsulator aids dosage design; Versatile drive offers quick setup
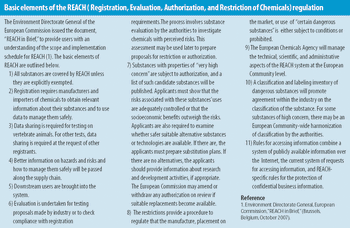
The European Union's REACH initiative has the potential to affect the flow of chemicals into the pharmaceutical suppy chain.

Show blasts off this month in Philadelphia with more suppliers, new trends, and real-world solutions.
A news roundup for March 2008.

My personal experience of lab automation is limited to supervising a peptide synthesiser back in the late 1980s. The machine was eye-wateringly expensive - but it was soon paying its way in terms of productivity and research publications. So if I were a stake-holder in a company pondering whether to invest in a well-designed gadget that could automate a routine operation, I'd say: 'go for it'.

Six sigma, process analytical technology (PAT) and related initiatives are driving greater use of statistical analysis methods to increase process understanding and improve manufacturing capabilities.

Also, Genmab will acquire PDL Pharma's Minnesota manufacturing facility, DSM Pharmaceuticals appointed Hans Engels president and business unit director, more...

Merck Capital Ventures, a subsidiary of Merck & Co., has invested $1.5 million to expand its service laboratory. The transaction with NanoImaging Services will add high-resolution, three-dimensional transmission electron microscope (TEM) capability, which allows biopharmaceutical researchers to see complex macromolecular structures they create with resolution as high as one nanometer.

The US Food and Drug Administration released a final guidance on protocol for testing sterile products.

Also, Biocon will acquire 70% of AxiCorp, ARIAD Pharmaceuticals promoted Richard W. Pascoe to the new position of COO, more...

Chesapeake to relocate and expand, AVI BioPharma appoints CEO, More...

Amira and GSK Form Agreement, Xenome Appoints Ian Nisbet CEO, More...

PharmTech's polls feature user feedback on issues facing the pharmaceutical industry.

Brief pharmaceutical news items for February 2008.

USP 467 Residual Solvents will take effect on July 1, 2008. But does the industry understand these specifications-and is it prepared?

A news roundup for February 2008.

The author suggests a route for nanotechnology's future in the pharmaceutical industry.

Can an overload of patent applications lead to the US' demise as a scientific leader?

Codexis Opens Budapest Lab, Allos Names Bruce K. Bennett VP of Manufacturing, More...

Actavis Acquires Site From Pfizer, Cardiokine Names President and CEO, More...

Novo Nordisk Drops Inhaled Insulin Product, Neurocrine Appoints CEO and President, More...

Crucell and Sanofi Pasteur Sign Agreement, WuXi Pharma Names COO, More...