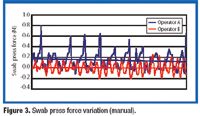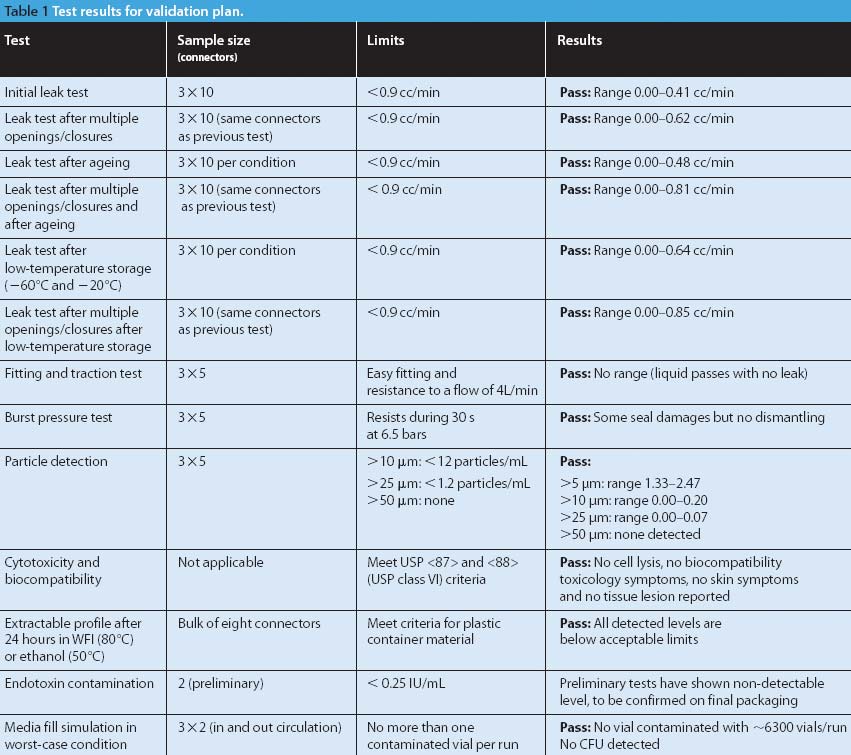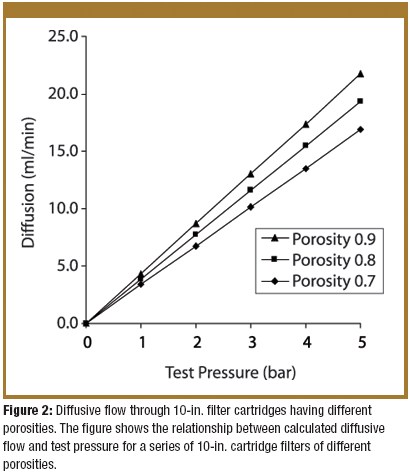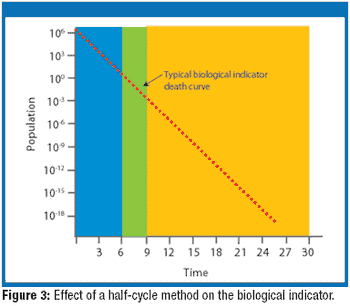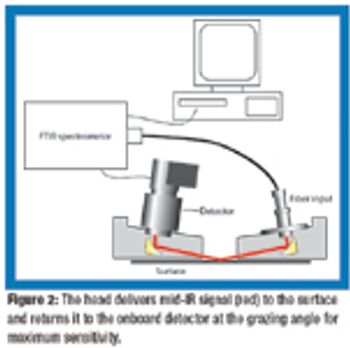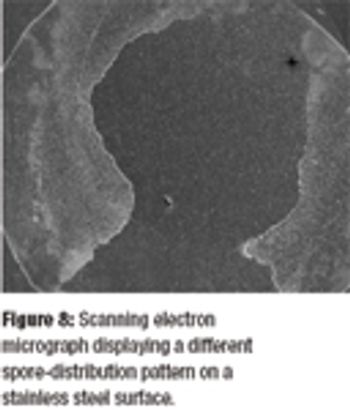
A biological indicator (BI) measures the effectiveness of the sterilization process to which it is subjected. Factors such as the test organism, the packaging, the culture material, and the test system all influence a BI's resistance. Carrier material is an often-overlooked factor that also influences BI resistance. The authors examine various solid and liquid carriers, describe their properties, and investigate how they influence BI resistance.