
Formulation residue, observer viewing distance, light intensity, viewing angle, observer viewing position, and observer-to-observer variability affect the ability to confirm the cleanliness of manufacturing equipment.

Formulation residue, observer viewing distance, light intensity, viewing angle, observer viewing position, and observer-to-observer variability affect the ability to confirm the cleanliness of manufacturing equipment.
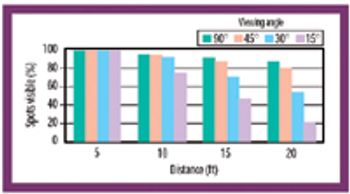
Formulation residue, observer viewing distance, light intensity, viewing angle, observer viewing position, and observer-to-observer variability affect the ability to confirm the cleanliness of manufacturing equipment.

This article provides an overview of the important factors associated with air handling systems within pharmaceutical and biopharmaceutical facilities. It provides information on the need for these systems, design considerations and advice on the approach to commissioning and qualification.

Possible cross-contamination issues should be eliminated at the early stage of the project. The project sponsor should ensure that all relevant personnel from the production, quality control, logistics, and maintenance departments, as well as engineering, are involved in the conceptual stages of a design.

New technologies and improvements to existing ones can reduce contamination risk in aseptic processing.

A ready-to-fill closed vial can improve aseptic filling quality and reduce process complexity.

The complete elimination of human-derived contamination is possible only with the elimination of human intervention.

The appropriate barrier system should be selected using a logical, risk-based approach, with awareness of all the possible sources of contamination.

Study results show that the state of a cleanroom clothing system–new or much used–influences the protection efficacy of the system.

The validation of alternative microbiological testing is an opportunity for a manufacturer to decrease the amount of time required for laboratory results.To properly validate these alternatives, a practitioner must first identify what is being studied. The regulatory effect on established product and process specifications and levels must be completely evaluated, as changing the method of analysis may well change the pparent number in the sample.

The sterility testing of samples from an aseptic process may be considered an entirely separate aseptic process that is subject to the same types of adventitious contamination as the aseptic process itself.

An increasing number of new compounds are being introduced into pharmaceutical pilot plants.The knowledge base for these compounds regarding their toxicities,physical handling, and cleaning is limited.The authors examine various approaches for addressing the cleaning validation of new compounds and discuss the role of determining appropriate visible residue limits.

A comparative study of three air samplers used for bioaerosol collection was performed to evaluate the average recovery of colony-forming units and assess the precision of each device.

The authors argue that chlorine dioxide (CD) is a safe and effective decontaminating agent that can be used for challenging applications.The effectiveness of CD gas for sterilizing complex isolator systems is studied.
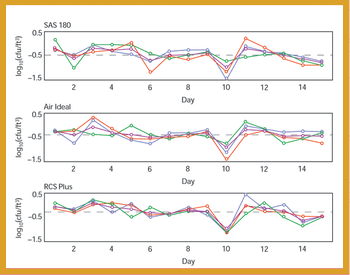
A comparative study of three air samplers used for bioaerosol collection was performed to evaluate the average recovery of colony-forming units and to assess the precision of each device.
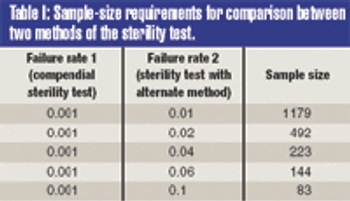
The validation of alternative microbiological testing is an opportunity for a manufacturer to decrease the amount of time required for laboratory results. To properly validate these alternatives, a practitioner must first identify what is being studied. The regulatory effect on established product and process specifications and levels must be completely evaluated, as changing the method of analysis may well change the apparent number in the sample.

This article outlines a comprehensive approach for organizing a firm's aseptic operations, including planning for routine and nonroutine interventions, establishing effective process simulations, and determining which vials to incubate.

A discussion of the validation and operation of two commercially available vapor-phase hydrogen peroxide decontamination systems is presented, based on a hands-on examination of both systems.

Evaluations have shown, in most cases, visual observations are sensitive enough to verify equipment cleanliness. An experiment was conducted to explore the possibility of using a visible-residue limit as an acceptable cleaning limit in a pharmaceutical research facility, including an evaluation of the limits and subjectivity of ?visually clean? equipment.

This article discusses the basics of sterile filter qualification and validation, with emphasis on bacterial challenge protocol development and testing. Reference is made to Technical Report no. 26 of the Parenteral Drug Association.

Single-use products enhance sterile filtration by making it easier to maintain sterility and reduce cross-contamination risks.

The authors suggest a design strategy for an aseptic process simulation that focuses on the basic repeating unit of the process, establishing alert and action criteria for the unit itself, and using worst-case simulations to establish routine operational parameters for the manufacturing process.

The authors examine the VHP resistance of microbial isolates recovered from controlled environments and compared them with commercially available biological indicators under various test conditions.

The limitation-of-risks (LR) method can be used as an engineering tool in risk assessment work for the identification, minimization, and evaluation of potential airborne risks, and for the identification of adequate monitoring points.

The role of microbial testing to ensure the sterility of aseptically filled sterile products is explained, from the product development phase to in-process monitoring to finished product testing.