
The US Food and Drug Administration has issued a not approvable letter to Merck & Co. in regards to their request for over-the-counter use of "Mevacor" (lovastatin) 20 mg.

The US Food and Drug Administration has issued a not approvable letter to Merck & Co. in regards to their request for over-the-counter use of "Mevacor" (lovastatin) 20 mg.

Members of Congress have strongly urged the US Food and Drug Administration to reconsider its proposed rule to amend regulations permitting companies to promptly update their drug and device labels with new safety information.

Codexis Opens Budapest Lab, Allos Names Bruce K. Bennett VP of Manufacturing, More...

Connecticut Attorney General Richard Blumenthal is investigating Schering-Plough (Kenilworth, NJ) and Merck & Co. (Whitehouse Station, NJ), according to a Reuters report.
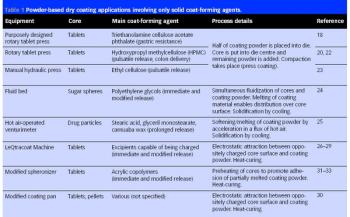
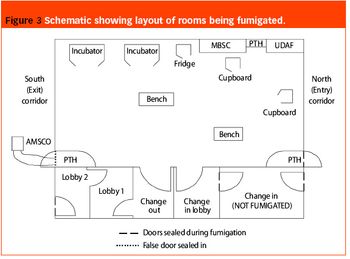
As part of a major project to design and build a new bulk vaccine antigen plant, the authors were asked to investigate and implement a suitable fumigation system for clean room decontamination. The facility was designed to handle and contain live influenza virus, and has clean room suites designed to containment levels CL2 and CL3 according to the Approved Code Of Practice and Guidance (ACOP, Control of Substances Hazardous to Health 4th Edition). From the outset, specific areas within the facility were identified as requiring fumigation and this formed part of the initial design brief.

Deciding where in the world to locate a new plant is a key decision for any pharma or biotech company - and there has never been more choice. Europe and the US now compete with the Far East and India, and what about the new EU states? Might Lithuania or Estonia turn out to offer advantages compared with France or Germany when it comes to finding the best place to take a new drug forward to the market place?

Robotization of end-of-line packaging systems enables manufacturers to maintain high production rates while accommodating flexible and varied product packaging requirements.

We are currently experiencing a problem with one of our tablet lines. While the tablets appear white immediately after manufacture, after a time many of the tablets begin to take on a yellowish appearance. Could this be an issue that surface analysis could help resolve?

In light of the rising price of stainless steel components and facility construction, pharmaceutical companies are increasingly using disposable components and systems in their manufacturing processes.

Featured products from this issue of Equipment & Processing Report

Actavis Acquires Site From Pfizer, Cardiokine Names President and CEO, More...

Roche and Ventana Medical Systems signed a definitive merger agreement, thus ending negotiations that began in June 2007.

The US Food and Drug Administration published a draft annex to its ICH Q8 Pharmaceutical Development guidance that clarifies that document?s key concepts.

Novo Nordisk Drops Inhaled Insulin Product, Neurocrine Appoints CEO and President, More...

Noven Pharmaceuticals received a warning letter from the US Food and Drug Administration stemming from an on-site inspection of the company?s manufacturing facility in Miami, Florida, concluded July 2007.

Pfizer and Hikal signed a contract manufacturing agreement in which Hikal will supply active pharmaceutical ingredients.

Crucell and Sanofi Pasteur Sign Agreement, WuXi Pharma Names COO, More...

The US Food and Drug Administration released a draft plan for modifying its information technology infrastructure. The plan follows the renewal of the Prescription Drug User Fee Act (PDUFA IV).

Richard Spoor, senior vice-president of global procurement at Merck & Co., Inc., discusses the company's strategy and progress made in its supply strategy that involves increased outsourcing and implementing lean-manufacturing principles in its manufacturing network. Pharmaceutical Technology's senior editor Patricia Van Arnum moderates.

President Bush signed HR 2764, making appropriations to the US Food and Drug Administration during FY 2008, which ends September 30, 2008.

Merck Sells Facility to Cherokee Pharmaceuticals, Inflazyme Announces Senior Management Resignations, More...

The $6.3 billion Indian pharmaceutical industry is at a crossroad. Aiming to be the international home for quality drugs, which could in itself propel India's market to $20 billion by 2015 according to recent estimates, the generic hothouse is clearly moving beyond its earlier low-cost mindset.
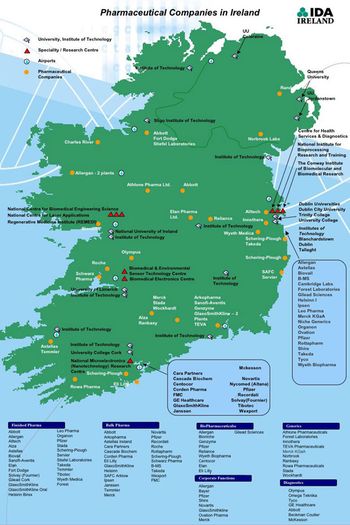
Ireland seeks to maintain a leading position in pharmaceutical and biopharmaceutical manufacturing as it also builds its base in research.

Singapore moves forward with a plan to diversify its life science investment with projects in biologics and drug discovery.