
Congress Focuses on FDA Inspections of Foreign Drug Facilities; Medical Students Oppose Big Pharma's Influence on Campus; FDA Revises Postmarketing Reporting Requirements

Congress Focuses on FDA Inspections of Foreign Drug Facilities; Medical Students Oppose Big Pharma's Influence on Campus; FDA Revises Postmarketing Reporting Requirements
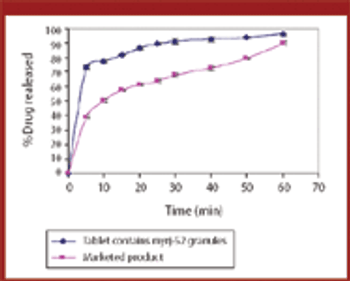
Various manufacturing techniques can improve a drug's solubility, thus increasing its bioavailability. The authors examined whether melt granulation can enhance drug solubility using meloxicam as the drug substance and myrj-52 as the binder.

A surge in capacity in contract microbial and mammalian cell-culture is underway to meet rising production needs for biopharmaceuticals.

The dynamics of the R&D pipeline could undercut recent CRO and CMO successes.

To keep up with applications, FDA promotes new testing and administrative methods.

In honor of Pharmaceutical Technology's 30th anniversary, the editors conducted a survey of 320 readers last spring to discuss industry advances and future directions. Here are some of your foward-looking responses.
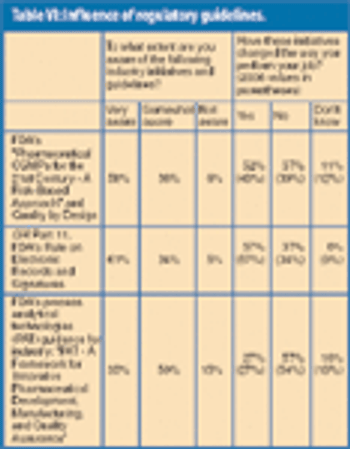
Nearly 1300 pharmaceutical employees provide insights into the issues most relevant to their jobs and the state of the industry workforce today.

Centrifuge processes many materials; New features for quality-management software; Unit simplifies powder discharge

As we get ready to head into the new year, chief executive and financial officers may be making their wish lists for 2008. Grow revenue. Increase pipeline. Establish new facility abroad. Acquire small-sized generics company. And so forth. But why not add something "green" to the list?Why not consider ways to help the environment while aiming for success?

Drug makers, drug inspectors, and drug consumers need to demand that new drugs be effective.

How do you answer an inspector's questions? How do you validate analytical methods? One book has answers for compliance professionals.

The "why" things are done in this industry is just as important as the "what"and "how."

Although the highly regulated pharmaceutical industry tends to be more like the tortoise than the hare when it comes to adopting new ideas, pharmaceutical packaging will be dramatically different 30 years from now in 2037.

"May you live in interesting times," goes the allegedly ancient Chinese curse. Well, these have been "interesting times" indeed for those working in the pharmaceutical industry.

Such an approach inevitably leads to increased front-end costs, but the advantages include better quality, reduced wastage and less product variability.

Incorporating quality and economy into downstream purification processes can expedite first-in-human clinical trials, product licensure and technology transfer. Experience in the chromatographic purification of biopharmaceuticals enables the use of downstream processing heuristics to produce target molecules in cost-effective processes suitable for regulatory scrutiny.
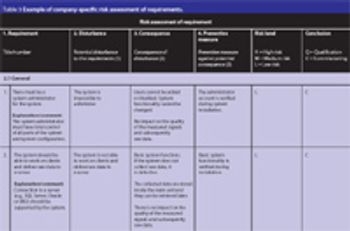
It is becoming evident that quality risk management within regulated, life sciences environments is a valuable component of an effective quality management system (QMS). A QMS provides a proactive and systematic means to identify, analyse, evaluate and control potential process and product quality issues during development, manufacturing, distribution and marketing throughout the entire product life cycle.

A well-devised QPP, which has been agreed on and signed by both parties, saves time and makes it easier to complete activities such as design, installations and tests.

Never has greater pressure been applied to pharmaceutical manufacturers. Shelf space competition for branded drugs has reached aggressive proportions and now even prescription drugs vie for pricing and delivery. Against this is a backdrop of ever-increasing downward price pressures, and a spectrum of progressively more stringent legislative and quality requirements. Finally, regional markets now demand different tamper evidence technology, anticounterfeiting measures and safeguards against interference by biological terrorists. Much of which points to the need for innovation in packaging - not just in terms of pack styles and sizes, but also cost.

Company and People Notes: ISP to raise prices, Patheon appoints CEO, more.

The US Food and Drug Administration has issued Ben Venue Laboratories a Warning Letter resulting from CGMP deviations found during an inspection of the company?s ?Propofol? injectable emulsion manufacturing facility earlier this year.

GlaxoSmithKline agreed to acquire Reliant Pharmaceuticals for $1.65 billion in cash.

Sanofi-aventis entered into an agreement with Chinese authorities allowing Sanofi to construct a vaccine-manufacturing plant in Shenzhen municipality.

Company and People Notes: Cambridge Major Labs acquires ChemShop; Helix BioPharma restructures senior positions, more.

Eli Lilly plans to invest $100 million in research and development in China over the next five years through Lilly Asian Ventures, a Lilly-owned venture capital firm that makes investments in Asia.