
Brief pharmaceutical news items for January 2008.

Brief pharmaceutical news items for January 2008.

What should contractors and their clients bear in mind when they collaborate on a project?

The traditional pharmaceutical business model is disintegrating, but the new model is not much different.

Process efficiency is measured not only by what is kept, but also by what is thrown away.

Editors' Picks of Pharmaceutical Science & Technology Innovations
News, world briefs, facility roundup, events and other items for January 2008.

As FDA implements new drug-safety policies, manufacturers will focus on quality and pricing.

QbD, PAT, design space, what's it all about? This seems to be a common industry response to FDA's directional push.

How much do you know about parallel trade? Perhaps you may have heard someone mention these words and have then switched off. In a sense, it's hardly surprising given the fact that most media coverage centres on interpretation of complex legal cases. By the time you reach the end of these types of articles, you can't work out what the mentioned companies were arguing about in the first place and on which technical details the case was judged. Yet, time after time, a legal ruling on a parallel trade issue rockets to the front pages of the pharmaceutical press and even, occasionally, the mainstream media.

The pharmaceutical industry in Asia is gearing up to be at the centre of the global market. Most pharmaceutical companies in the region expect this shift to happen fast. Not only is Asia set to become the largest pharmaceutical market in the world, but many Asian territories will be the powerhouses of the industry. By 2020, the worldwide pharmaceuticals market could be worth around $1.3 trillion, with China being the second or third biggest market.

While it's unlikely that Al Gore's Nobel Peace Prize will gain him the US Presidency, the award has put global warming firmly in the spotlight (as if it wasn't there already). Reducing its carbon footprint is among the many goals Big Pharma has for the coming year - and beyond. Many energy consumption issues are common to all industries and individuals, such as putting curbs on business travel, bringing renewable energy sources on board and switching off lights in unoccupied rooms, but there are other issues that are specific to our industry. For example, asthma inhalers emit greenhouse gases and many pharmaceutical syntheses use toxic solvents.
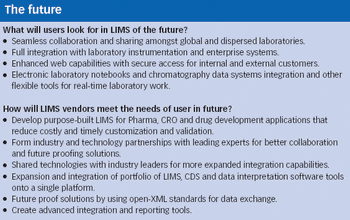
Historically, the main purpose of laboratory information management systems (LIMS) has been to track and manage samples in the laboratory. LIMS originated nearly 30 years ago as a rudimentary method of automating manual, error-prone processes in the laboratory and, with the growth in adoption of technology, became the de facto benchmark for laboratory control and management.

However, companies should always be prioritizing prevention, elimination and reduction over recycling and recovery as the most effective ways of making resource efficiency savings.

Forward-thinking manufacturers are recognizing the need for a more sustainable approach to containment design.

Millipore and Novozymes Form Alliance, Applied Biosystems Names President and CEO, More

Merck & Co. initiated a voluntary recall of 11 lots of its Haemophilus influenzae type B vaccine, Pedvaxhib, and two lots of its combination Haemophilus influenzae type B/ hepatitis B vaccine, Comvax.

Company and People Notes: West to reduce workforce, Eli Lilly's CEO and chairman to retire, more...

After a negative year in the news, China has finally agreed to improve the safety of its drug and food exports, according to the US Department of Health and Human Services.

The US Food and Drug Administration posted on its website a Warning Letter it sent to Wyeth Pharmaceuticals regarding a journal advertisement for the company?s ?Effexor XR? antidepressant.

The US Food and Drug Administration issued a rule that clarifies its requirements for current good manufacturing practices for aseptic processing, water standards, and verifications standards.

Company and People Notes: Eisai Acquires MGI Pharma, Ceregene Names CEO, More

Perfect pumps for bio-processing and high-flow sanitary duties Watson-Marlow Bredel's 720 series peristaltic pumps are the perfect pumping solution for single-use or fixed bioprocessing systems. Featuring a smaller footprint than other pumps with a similar flow rate, 720 pumps provide low shear pumping up to 4,000 litre/hour and use sanitary LoadSure pumping elements for one minute maintenance.

A unique delivery process could mean faster, cheaper and more effective vaccination procedures.

After a good year of economic output growth, the EU chemical industry is losing momentum and a large drop in growth is expected for 2008 because of a cooling down of the world economy.

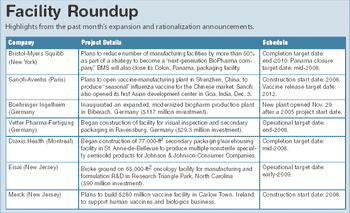
Bristol-Myers Squibb outlined a comprehensive review of its operations and a strategy to improve top-line growth, a plan that involves staff reductions and rationalization of its manufacturing plants.