
CMC Biologics will manufacture monoclonal antibodies (mAbs) and provide process development services for the PATH Malaria Vaccine Initiative.

CMC Biologics will manufacture monoclonal antibodies (mAbs) and provide process development services for the PATH Malaria Vaccine Initiative.

Catalent selects advisory board members to complement Redwood Bioscience Scientific Advisory Board.

Contract research, development, and manufacturing organizations (CROs, CDMOs and CMOs) are embracing lean manufacturing, while Big Pharma is applying it, not so much for inventory management, but to improve supply chain visibility and control.

FDA issues guidance regarding fees for drug compounding outsourcing facilities.
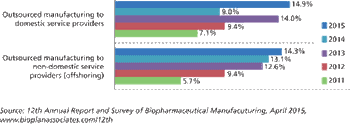
Biopharma companies are outsourcing more jobs to cut costs.

The YDP30 printer from Sartorius automatically performs thermal transfer or direct thermal printing, without the need to change settings, to meet requirements for fade-resistant printout in regulated areas, such as quality-control laboratories.

Create an efficient global labeling strategy that is compliant with both electronic and paper-based package-insert requirements.

Contract development and manufacturing organizations join forces to address legislative, regulatory, and business concerns in the Pharma & Biopharma Outsourcing Association.

What if the expanding pipeline isn’t enough to fuel CMO growth?

Driven by competitive pressures and using models pioneered in other industries, pharmaceutical companies are extending collaboration efforts well beyond their walls.

Ignoring a contract partner’s ability to handle highly potent APIs (HPAPIs) safely may have serious consequences. Drug owners and contract service providers alike must understand the complexities and liabilities involved in working with HPAPIs.

After a long wait for the new elemental impurities guidelines, the bio/pharma industry must now look ahead to implementation and take action.

Cloud computing has made it easier for pharma companies and their contract partners to gain visibility into their combined supply chains.
Within the past few years, key players have left the sterile manufacturing business. Can new technology and investment revitalize this critical market?

Currently, pharma has only scratched the surface of the potential value of repurposing drugs, says consultant Hermann Mucke. Contract service partners are playing a more prominent role, but could play an even larger one in these efforts.

Sponsors and contract partners alike should not assume that upcoming US federal deadlines will be as elastic as California’s were.

Investment in Powdersize adds particle-size control solutions to Xcelience’s capabilities.

Lupin acquires businesses in Germany, the US, Russia, Brazil, and South Africa.

Avista grows its contract services business, and Scynexis focuses on antifungal development.

AMRI expands its API portfolio and European presence through acquisition of Gadea’s Crystal Pharma Group.

Evans Analytical Group expands into the pharmaceutical/biopharmaceutical industry with acquisition of ABC Laboratories.

Catalent’s licensing of Excelimmune’s antibody combination therapy platform can enable the manufacture of multiple recombinant antibodies in a single batch culture.

Keith Moore will support Metrics Contract Services as vice president of analytical services.
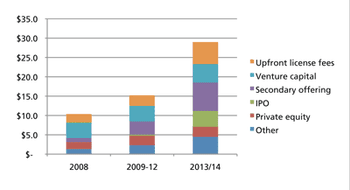
While all market signs are pointing up, memories of past setbacks may discourage from expanding capacity.

The addition of a new manufacturing line at Lonza’s Portsmouth, NH site enables Alexion to add dedicated product supply for 10 years.