
New instrumentation at the SGS Mississauga, Canada facility advances testing capabilities for packaging and container testing.

New instrumentation at the SGS Mississauga, Canada facility advances testing capabilities for packaging and container testing.

Closing the summer of 2015, the ChemOutsourcing conference examined contract services from both a sponsor’s and a CMO or CRO’s perspective

The two companies will combine life-sciences marketing and a consulting firm to create a life sciences-centered consulting service.

Scottish injectable-drug manufacturer Symbiosis Pharmaceutical Services plans to expand its sterile filling facility.

CDMO Aesica Pharmaceuticals introduced a new service approach in which the company will taks on the management of other CDMOs, while serving as the single, central point of customer contact, the company announced in an Oct. 14, 2015 press release.

In the past, contract manufacturing organizations (CMOs) and contract development manufacturing organizations (CDMOs) have been focused most on achieving scale, reaching new clients in new geographic regions, and adding services that were previously unavailable, according to Gil Y. Roth, president of the Pharma and Biopharma Outsourcing Association. In a new report from CPhI, Roth explicates how consolidation has changed how CMOs position their services to clients and how CMOs add value to their businesses.

Upgrades to Mettler Toledo’s weighing and dosing equipment increase productivity using lean laboratory principles.

Cell Therapy Catapult will provide manufacturing scale-up services to enable Asterias’ future clinical trials and commercial supply for Asterias’ allogeneic dendritic cell immunotherapy, AST-VAC2.

Contract manufacturer PiazaBio announces services for Western Pharma companies seeking to enter the China market.

Merck Millipore’s collaboration with celares GmbH launches pegylation services for protein-based therapeutics.
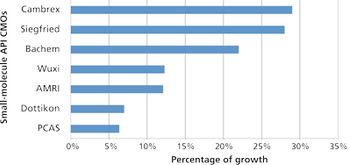
Despite emergence of biologics, small-molecule APIs benefit from industry growth.

Vetter plans to invest approximately 300 million Euros during the course of five years to expand drug product manufacturing and logistic services in Germany; upgrades will include an improved RABS system for aseptic processing.

Sterigenics International will triple West Memphis sterilization capacity with $15 million expansion.

The Grünenthal Group’s Tecnandina manufacturing plant in Ecuador has received the country’s first certification from the Regulatory Authority of Brazil.

The CDMO is striving to become a market leader in the development and manufacturing of antibody-drug conjugates.

Pharma development and manufacturing firm Patheon announces new tagline and logo.

Cobra Biologics and the University of Manchester announce a collaboration to improve industrial scale-up of mammalian cell bioprocessing.

Abenza acquired biopharmaceutical CDMO PacificGMP and expanded the company’s San Diego facility.

PharmSource forecasts slow growth in a new report on the outlook for the contract manufacturing industry.

SGS Life Science Services announced that its facility in Fairfield, NJ, has been upgraded to be Biosafety Level 2 (BSL-2) compliant, according to the Centers for Disease Control and Prevention (CDC) guidelines.

The partners will collaborate on a project to capture and integrate clinical trial data metrics on investigational cancer medications.
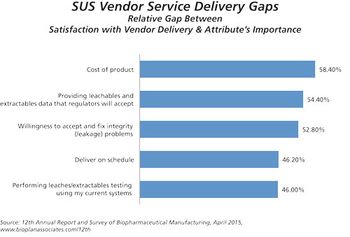
Suppliers indicate prices for single-use equipment are likely to increase.

Kason’s Vibro-Bed fluid-bed agglomerator is equipped with imbalanced-weight gyratory motors and mounted on a spring suspension.

Mayne Pharma plans to double oral-dose manufacturing space and expand contract services.

Recipharm will be responsible for the supply of the remaining clinical-trial material and ongoing future commercial supply of RHB-105, the lead drug candidate developed by RedHill for the treatment of Helicobacter pylori bacterial infection.