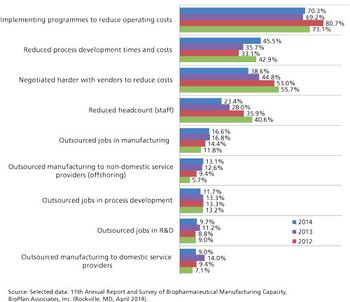
Outsourcing is taking on a greater role in the biopharmaceutical manufacturing industry.

Outsourcing is taking on a greater role in the biopharmaceutical manufacturing industry.

Advanced analytical methods are speeding up the targeted evaluation of potential viral contaminants.

The authors present a validation study of an analytical method for the simultaneous determination of nine human cytokines in human K2-EDTA plasma.

The authors describe a rapid, single-point calibration approach for ICP–MS analysis of raw materials used in drug product manufacturing.

European CDMOs want into the US market, but entry options are limited.

The trend of exits from the CMO industry looks to be gaining momentum.

Multidirectional collaboration is critical for the new pharma business model; cloud-based information services can offer a communications alternative.

John S. Ross, executive vice president at Metrics Contract Services, provided commentary on the evolving role of contract services in solid/semi-solid dosage development and manufacturing.

Experts at Catalent Pharma Solutions provided commentary on the evolving role of contract services in analytical services, biologics drug development and manufacturing, and solid/semi-solid dosage development and manufacturing.

Experts from PPD provided commentary on the evolving role of contract services in analytical services.

Franco Negron, vice president of North America operations and integration at Patheon Inc., provided commentary on the evolving role of contract services in solid/semi-solid dosage development and manufacturing.

Mark Rogers, senior vice president at SGS Life Science Services, provided commentary on the evolving role of contract services in analytical services.

Experts at Dr. Reddy's CPS provided commentary on the evolving role of contract services in biologics drug development and manufacturing, and solid/semi-solid development manufacturing.

Peter Soelkner, general manager at Vetter Pharma International GmbH, provided commentary on the evolving role of contract services in parenteral drug development and manufacturing.

Andrew Strong, president and CEO of Kalon Kalon Biotherapeutics, provided commentary on the evolving role of contract services in biologics drug development and manufacturing.

Ron Connolly, vice president of commercial operations at Frontage Labs, provided commentary on the role of contract services in analytical services.

Experts at Xcelience provided commentary on the evolving role of contract services in analytical services and solid/semi-solid development manufacturing.
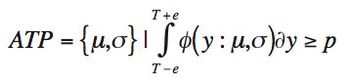
The quality-by-design principles used to control process variability are equally important to measurement systems because process variability includes contributions from measurement system variability. The authors use real-life examples from drug development projects to outline how an understanding of chromatographic measurement system variability might be achieved.

Experts from Baxter BioPharma Solutions provided the following commentary on trends in bio/pharmaceutical development outsourcing.

Annual study shows geographic proximity not a factor in CMO selection.

John Sandles, Business Analyst for Fujifilm Diosynth Biotechnologies, provided commentary on the evolving role of contract services in biologics drug development and manufacturing.

Changing regulations are impacting the identification and monitoring of variable materials in excipients.

Biopharma manufacturers must reduce the risk in their complex supply chains

Annual study shows CMO technical expertise is not enough.

Shrinking facility size, growth of biologics, and emerging market demand influence pharma investments.