
Xcellience receives approval to handle both analytical and manufacturing of DEA Scheduled products.

Xcellience receives approval to handle both analytical and manufacturing of DEA Scheduled products.

As an affiliate member, TraceLink brings serialization expertise to the Pharma & Biopharma Outsourcing Association.

Patheon cites expanded API services with acquisition of IRIX Pharmaceuticals.

Steve Nole joins Grand River Aseptic Manufacturing as director of manufacturing.

WellSpring Pharma Services has announced the appointment of David Mayers as president.

A facility expansion adds space for production of Repligen’s tangential flow system.

The Pharma & Biopharma Outsourcing Association announced its support of legislation that would preserve FDA user fees from sequestration.

SGS Life Science Services adds analytical methods to identify amino acid impurities in bio/pharmaceutical manufacturing at facility in Germany.

Novasep has entered into an agreement with Celladon to provide scale-up and pre-validation studies for the drug substance for MYDICAR.

The fast growth of the global biopharmaceutical market has prompted global pharmaceutical and biotechnology companies to increase their R&D investment in biologics.

Protecting workers, patients, and the environment requires advanced technologies.
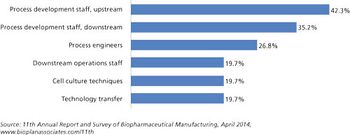
Is there enough talent to go around?

The company has increased capacity for cold storage and controlled drug substances handling at its European facilities.

SGS has invested in additional modules for its COBAS 6000 analysis system in a move to expand its biomarker analytical capabilities at its Poitiers facility in France.

Careful selection of a contract packager should include interviews, surveys, and on-site visits.

The agency releases five draft guidance documents related to drug compounding and repackaging.

Market forces may limit the success of CMOs.
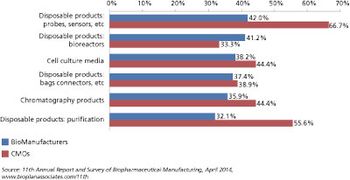
Biomanufacturers are seeking more innovation and expertise from their CMO partners.
Whether outsourcing or developing cell therapies in-house, success demands a focus on quality, cost of goods, and sustainability from the start.

CMO executives share their opinions on where outsourcing is going and what is driving market change.

As CMOs shed their old toll processing role, sponsors can expect the right questions, proactive communication, and a firm grasp of risk management and tech transfer from contract service providers.
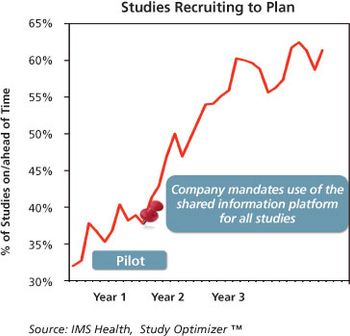
Data sharing and a common data model can improve CRO partnerships and trial results.

Better equipment and automated processes will be key. So, too, will the right way of approaching outsourcing relationships.

The company has installed and validated new instruments for structural analysis of proteins.

EMA Recommends Suspension of 700 Drugs Tested at GVK Site GVK Biosciences argues that EMA’s recommended suspension of 700 drugs is disproportional to reported infractions. The European Medicines Agency (EMA) has issued a recommendation that 700 medicines authorized for use in the European Union (EU) should be suspended, based on concerns about how GVK Biosciences, a contract research organization in Hyderabad, India, conducted clinical studies. GVK Biosciences, in response, argued “the action is unprecedented and highly disproportional.”