
A new SGS Life Science Services laboratory outside Paris is designed for bio/pharmaceutical quality control testing.

A new SGS Life Science Services laboratory outside Paris is designed for bio/pharmaceutical quality control testing.

Presenters at IVT's Microbiology Week discussed best practices and recent guidance publications for microbial control in sterile and non-sterile pharmaceutical processes.

Catalent’s Singapore facility is awarded GMP certification.

Vetter completes on-site expansion activities of visual inspection and in-process control at Chicago facility.

Huber Kältemaschinenbau will be exhibiting temperature control solutions for the research laboratory and process industry at ACHEMA 2015.

Porvair has developed a 2-ml 96 round-well deep-well plate that enables scientists to use the full 2.00 ml well capacity.
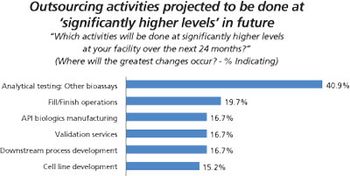
Biopharma companies on both sides of the Atlantic ship more of their assay testing to outside service providers.

ProMetic Life Sciences will use manufacturing and plasma fractionation processes at Emergent BioSolutions’ Winnipeg, Canada facility.

Clinical supplies facility in Frankfurt, Germany will triple Theorem Clinical Research’s current clinical supplies capacity.

Grand River Aseptic Manufacturing named one of Michigan 50 Companies to watch.

This new pharmaceutical stability storage facility will enable the company to expand its analytical and formulation offerings to the pharmaceutical and biotech industries.

EMA confirms its recommendation to suspend medicines approved based on GVK Biosciences studies.

The use of disposables requires careful consideration and planning.

FDA issues a Form 483 following inspection of Impax Laboratories' Hayward, CA facility.

Capsugel extends inhaled biotherapeutics delivery capability to Phase 2 clinical trials.

While the United States and Europe still dominate, CMOs and CROs based in emerging markets continue to capture market share.
The rapid testing of biologic raw materials can lead to greater efficiency.

Pharmaceutical companies should take into consideration intellectual property protection when outsourcing the packaging of their products.

CDMO Vetter produces identifiable labeling for a top-ten pharmaceutical company.

IDT Biologika receives 2015 Facility of the Year Award or facility integration from ISPE.

Stability testing and UPLC capabilities highlight expansion at Mumbai, India facility of SGS Life Science Services.

INTERPHEX 2015 is under way and Pharmaceutical Technology and BioPharm International are in the middle of the action!

The past six months has seen some major changes to the sterile manufacturing landscape in Europe. There have been a number of exits and acquisitions that have no doubt grabbed headlines, but has anything really changed?

A presentation by Jim Miller will offer a detailed review of the contract services landscape.

Rentschler Biotechnologie expands European manufacturing capabilities with GE Healthcare Life Sciences bioprocess technologies.