
Capsule filler

Capsule filler

Speedy Filler Produces 200,000 capsules/hr

Tablet Press

Tablet Press with Fastest-in-Class Changeover

Discovery of split and broken tablets led to drug recalls.

I Holland announces service that predicts solutions for tablet sticking problems.

ALpHA G Capsule Filter for Single-Use Systems

Training, calibration, and preventive maintenance help prevent over-and under-weight tablets. The author discusses causes of off-weight tablets and best practices for tableting.

Operator training, preventive maintenance, and regularly scheduled calibration help prevent the manufacture of off-weight tablets.

Excipients for lowering formulation costs and improving lipid-based formulations and the use of melt-spray-congeal microsphere sachet technology for targeted controlled release attract attention at the 2013 American Association of Pharmaceutical Scientists Annual Meeting.

Tablet Tooling Coating Reduces Formulation Sticking

Analytical tests, correlated with statistical techniques, are used to predict material behavior.
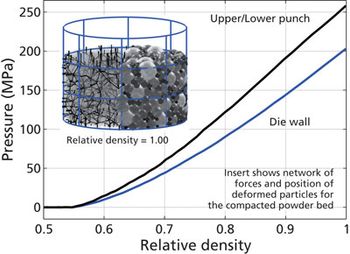
Screening methods and predictive models address tenacious tablet-sticking problems.

Pliva?s new Oral Solid Forms Facility will increase Pliva's production capacities for tablets and capsules.

Capsule design was developed for patients who have difficulty swallowing.

Interview of Detlev Haack, head of R&D, Hermes Pharma on the characteristics, benefits and manufacturing Methods of effervescent tablets

Stephen Tindal, Director Softgel Formulation at Catalent, talks with Pharm Tech about manufacturing issues with soft gels.

Kansas State University to build Bulk Solids Innovation Center in Salina, Kansas.
HERMES PHARMA has pioneered user-friendly dosage forms. Our effervescent and chewable tablets, lozenges, orally disintegrating granules and instant drinks are convenient to use, easy to swallow and taste good.

Analytical methods are being used to troubleshoot tablet-sticking problems and to develop screening methods and predictive models to more quickly find solutions.

An industry leader since 1933, Catalent develops and manufactures over 80% of the world's Rx softgel products with 200+ products on the market in 80+ countries.

Boehringer Ingelheim initiates a recall due to the potential for extrinsic foreign particles in the API used to manufacture Spiriva Handihaler (tiotropium bromide inhalation powder) capsules.

Foam granulation is much easier to control under mechanical dispersion conditions, which is where most industrial processes operate.

Sanofi and POZEN sign a license agreement for the commercialization of omeprazole and aspirin combination product.

The Bosspak VTC 100 electronic tablet and capsule counter from Romaco?s is designed to fill pharmaceutical solids or food supplements into bottles at high speed.